This is adamantane:
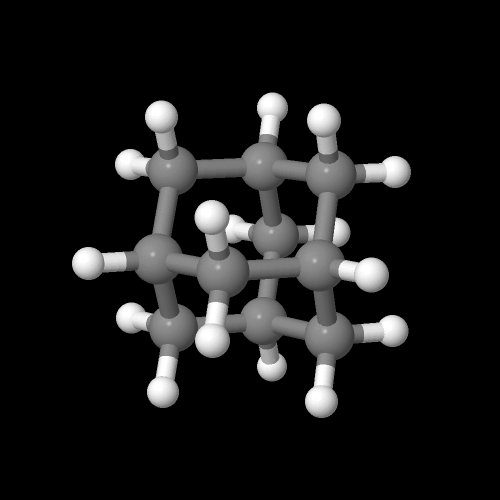
Click on the image to go to a 3D model you can zoom
and rotate and do lots of cool stuff with.
So what's up with this strange molecule?
Adamantane is an unusual molecule that can actually be found in high abundance in some oil wells. It can also be made from the hydrogenated dimer of cyclopentadiene by a simple rearrangement process using heat and a catalyst. That's to say, most people think adamantane is pretty expensive, but it's actually not. Pound for pound, in fact, it does things no other pendent group on a polymer can possibly do.It's cousin to molecules containing two, three or more adamantanes fused together, which can also be found in some oil wells. They're collectively called "diamondoids" since they're actually natural precursors to diamond. They just haven't been exposed to as much heat and pressure to finish the job to being the diamonds we find deep underground. In one sense, then, adamantane is the monomer and repeat unit of diamond. Which means what, that diamond is actually a polymer?
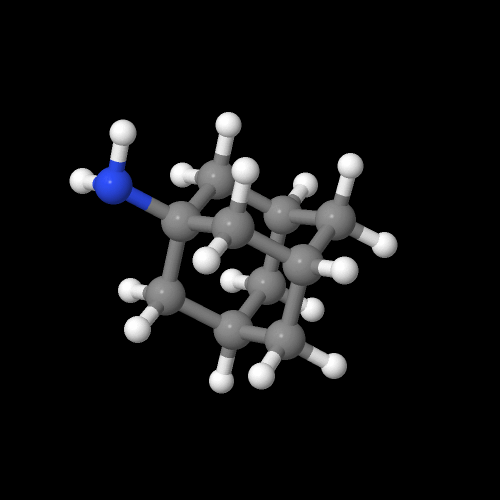
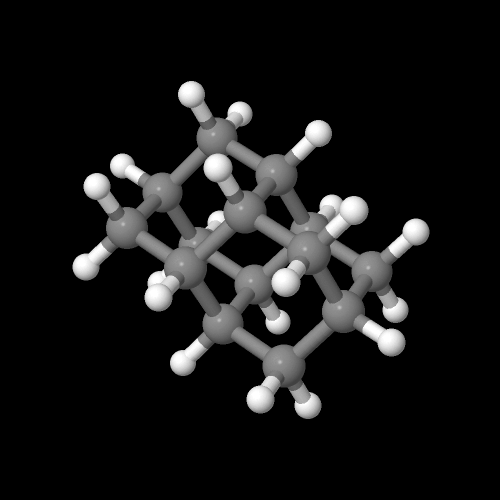
Click on an image to go to a 3D model you can zoom
and rotate and do lots of cool stuff with.
And here's another interesting fact about adamantane in all it's glory and unusual properties: there are several amine derivatives which have antiviral activity. That is, they are some of the few chemicals we know of that can actually fight viruses. In fact, the simple amine-substituted adamantane (shown above) not only fights viral infections, it helps with Parkinson's disease as well, for reasons no one seems to fully understand. But hey, if it works, let's use it while we figure out how so we can make it work better, no?
And if you find the biological activity of adamantanes interesting, be aware that it's been incorporated into or a major part of hundreds of small molecules. These function either by themselves or as part of a catalyst complex, to name two. Take a look here to get some idea of the many derivatives that have already been made...
So what's so interesting about adamantane by itself? Sure, it looks like the repeat unit of that massively crosslinked material we call diamond, but does it do anything useful for polymers? Great question, glad you asked, and it turns out the answer is a resounding "YES!" And now you might ask "How does it do whatever it does you're so excited about?"
Well, that's the whole point of this page, now isn't it? Let's start with a simple polymer made from adamantyl acrylate. This acrylate is made by reacting adamantol, the hydroxy-substituted adamantane, with either acryloyl chloride or acrylic anhydride (see below). Using a base and catalyst helps, but in any event, the monomer is easy to make and purify. In fact, you can make a lot of monomers for a whole lot of different kinds of polymers from adamantol, adamantamine and bromoadamantane, all simple derivatives easy to make.
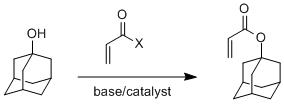
And here are images of 3D versions of adamantyl acrylate and methacrylate. Click on the images to pop up the actual 3D versions you can rotate and zoom in on. We'll get to their polymers next.
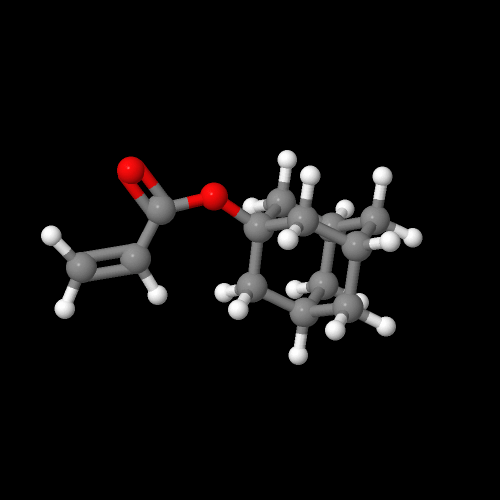
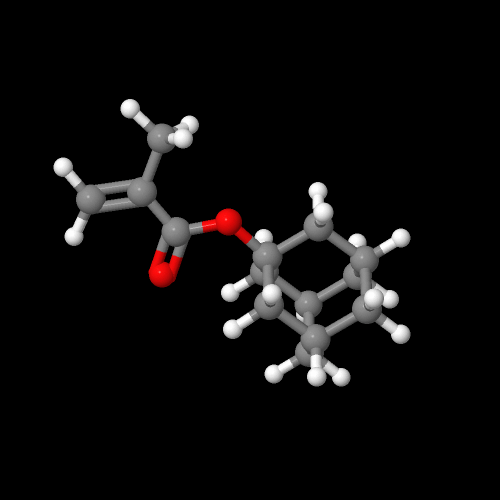
Click on an image to go to a 3D model you can zoom
and rotate and do lots of cool stuff with.
So those pendent adamantyl groups are pretty big, wouldn't you say? Well, ok, they're not all THAT big, but what effect do they actually have on polymer properties? Let's compare the methyl ester polymers with the adamantyl ester ones for both acrylates and methacrylates. First, though, you might want to take a look at the 3D models of the polymers from these two monomers. Click here to see the acrylate polymer and here for the methacrylate.
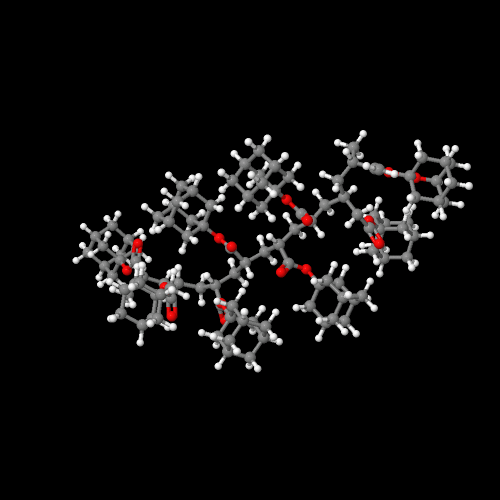
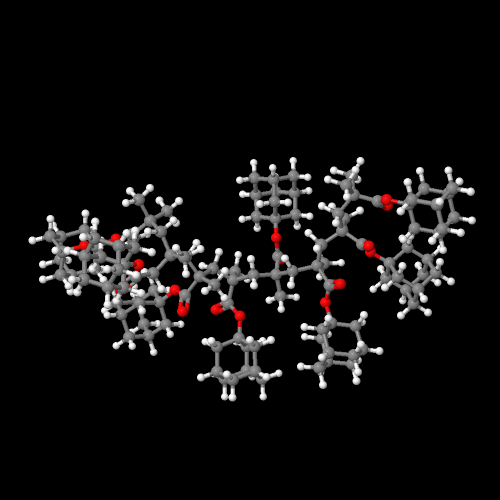
Click on an image to go to a 3D model you can zoom
and rotate and do lots of cool stuff with.
And for comparison, let's also look at a polymer that should NOT have been made, at least not easily. There's an important point here, but I'll save that for later. Below is the structure on the left for a relatively ignored family of monomers, the dialkyl fumarates. These diesters have parts in common with alkyl methacrylates; namely, a vinyl group with a conjugated ester attached. Fumarates, though, are 1,2-disubtituted rather than 1,1-disubstituted. And that makes for problems during free radical polyaddition. In fact, polymerization just doesn't happen for the free acid, the methyl ester and various linear alkyl esters.
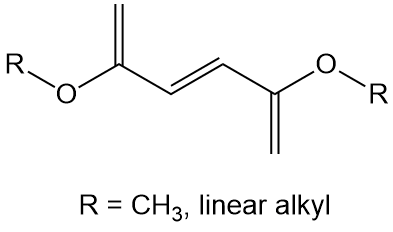
Click on the right image to go to a 3D model you can
zoom and rotate and do lots of cool stuff with.
Interesting enough, some intrepid scientist, undeterred by prior failed attempts, decided to try free radical polymerization of the di-t-butyl ester. Lo and behold (to coin a phrase), it worked. Not only worked, but gave high molecular weight polymer. On further theoretical and experimental examination, it was discovered that a surprising kinetic effect was evident here. It was not that the t-butyl groups enhanced the propagation step to allow polymerization, and in fact, propagation was not facilitated at all. BUT (and it's a big but!)what was impacted enormously was the termination process. Free radicals are never very happy, so if propagation can't happen, sooner or later some kind of chain transfer or termination process will. With the steric hindrance caused by the t-butyl groups, though, the radical couldn't get near each other to couple or disproportionate or do much of anything else. So as a last resort, the terminal radical carbon would eventually find a fumarate monomer carbon to react with. Overall, the propagation occurs by default, so I guess we could term this sterically-induced process "default polymerization."
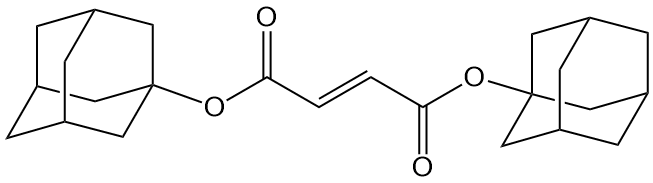
Back to adamantanes, then. Having discovered this unusual ability for the di-t-butyl fumarate to polymerize, we thought to ourselves, "Selves, adamantane is even bigger and bulkier than t-butyl..." Well, you can see where we're going. So a gullible post-doc was convinced to make the diadamantyl fumarate (shown above) to try polymerization with. Lo and behold (ok, I'll stop with the ancient phrases), it polymerized just fine- no side reaction, no or little termination, and high molecular weight linear polymer. Bingo! And for your edification, the polymer image is given below: click on it to load the 3D version you can explore in more depth.
Click on the right image to go to a 3D model you can
zoom and rotate and do lots of cool stuff with.
And now the burning question in the front of your mind (the back is already occupied with thoughts of something else): how about physical properties such as solubility, glass transition temperature, crystallinty and mechanical properties? Actually, that's four questions all rolled into one, but good ones nonetheless.
 Go to a list of adamantane compounds
Go to a list of adamantane compounds
 Return to the Glass Transition Page
Return to the Glass Transition Page
 Return to Macrogalleria Directory
Return to Macrogalleria Directory