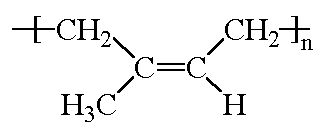
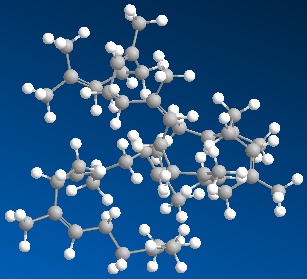
The model on the right above is of the cis polymer you can
view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
One of the most well known natural polymers is polyisoprene, or natural rubber. Ancient Mayans and Aztecs harvested it from the hevea tree and used it to make waterproof boots and the balls which they used to play a game similar to basketball. It is what we call an elastomer, that is, it recovers its shape after being stretched or deformed. Normally, the natural rubber is treated to give it crosslinks, which makes it an even better elastomer.
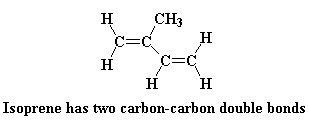
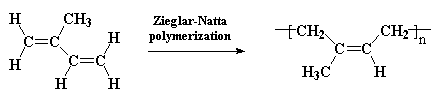
Polyisoprene is diene polymer, which is a polymer made from a monomer containing two carbon-carbon double bonds. Like most diene polymers, it has a carbon-carbon double bond in its backbone chain. Polyisoprene can be harvested from the sap of the hevea tree, but it can also be made by Ziegler-Natta polymerization. This is a rare example of a natural polymer that we can make almost as well as nature does.
Here's some historical information. During World War II, the Japanese cut off natural rubber supplies for all the rest of the world. And since natural rubber was the predominant form in use at the time, the Allies were stuck. Imagine an airplane without rubber gaskets and hoses, not to mention tires that absorb shock during landing. Now think about trucks, tanks and jeeps with the same problems. Wonder what the ride would be like in a truck going 60 with steel wheels?
So science came to the rescue! A massive government funded effort was mounted in the US that very quickly lead to synthetic rubber. First out of the block was polyisobutylene. It was a pretty good rubber (and still is) but mainly for the unusual fact that it doesn't pass gas (sorry). It's the only commercial polymer that will hold air inside a tire for weeks, even months, at a time. Natural rubber can't do this, so one outcome of the war effort was a solution to the major problem of having to air up your tires every week.
Ok, the war ended, natural rubber was available to the entire world again. Production zoomed! But then, so did the effort to make other synthetic elastomers like polybutadiene and polychloroprene. The latter was another serendipitous discovery like that of PIB: polychloroprene (also called neoprene) was able to NOT swell in contact with gasoline, diesel or motor oil. Another fantastic advance in polymer science!
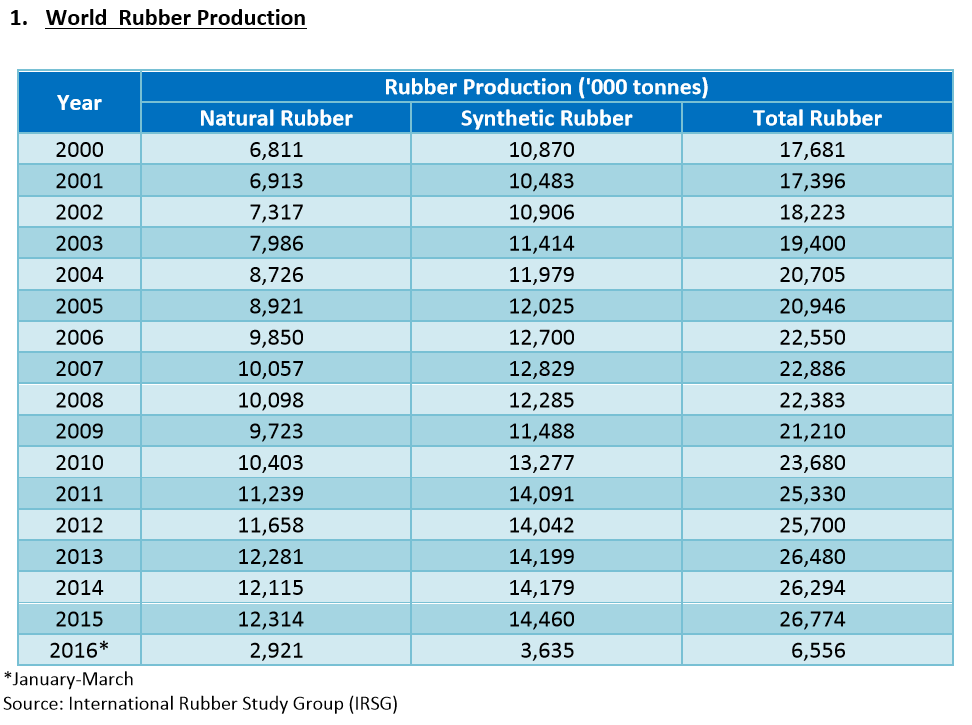
So what's the status today of natural versus synthetic rubber? They share the market, and it's a huge one at roughly 26 million tons per year. According to the chart above, published in 2016, roughly 54% of the world-wide production is of synthetic rubber of various kinds. That leaves a whopping 46% to natural rubber. And the great thing about that is that natural rubber is a renewable resource, coming from that most important kind of renewable resource, trees. Way to go, Mother Nature!
This is what the isoprene monomer looks like:
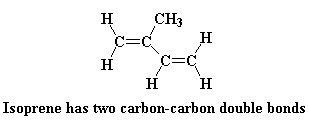
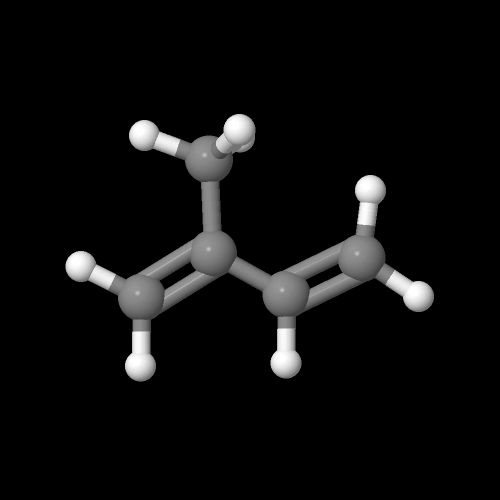
The model on the above is an image of the pdb model you can
view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
Tested Polyisoprene Syntheses
So maybe you've tried making polybutadiene and that went so well, you now really want to make your own polyisoprene. Well, it turns out, we actually have not one, but TWO procedures. One gives the mostly cis repeat unit polymer, the other mostly trans. Volatiles and flammable catalysts require extreme care in the lab to be safe: just do it very carefully!The two methods use two different transition metal catalysts to give two different isomeric PIP's. The first gives the cis isomer: click here to see the procedure and here to download a copy.
The second method using a different catalyst and conditions gives the trans isomer. Needless to say, the two polymers have very different properties. Click here to see the procedure and here to download a copy.
Other polymers used as elastomers include:
- Polybutadiene
- Polyisobutylene
- Poly(styrene-butadiene-styrene)
- Polyurethanes
- Polychloroprene
- Silicones

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
