
2/8/1999
Emulsion polymerization of monomers capable of stabilizing a free radical (e.g. Styrene) and monomers using redox initiators (e.g. Acrylates) has been found to yield higher MW polymers due to the lack of termination occurring in the micelles. Also, one can increase the degree of polymerization and the rate of propagation by increasing the amount of surfactant. However, changing the initiator concentration should not have any effect on the MW of the chains as will be discussed later.
Dilute solution viscometry of poly(ethyl acrylate) (PEA) was utilized to find the intrinsic viscosity of the polymer, the measure of how much a polymer can increase the viscosity of a solvent (acetone) at a certain temperature (25oC). The Mark Houwink equation, h=KMa illustrates the relationship between intrinsic viscosity and molecular weight. This relationship was investigated in this lab and was used to find the molecular weight of the PEA sample using the Mark Houwink K and a values for PEA at the aforementioned conditions.
Size exclusion chromatography (SEC) is a characterization technique used to find the number and weight average molecular weight (among other molecular weights) and the polydispersity (PDI) of a polymer sample. In this lab polystyrene (PS) was analyzed. SEC involves the fractionating of a polymer sample by trapping smaller chains in the pores of the column medium. Thus the speed at which the chains go through the column depends on their size. The typical PDI of emulsion polymerized PS is about 1.5.
Experimental
The emulsion polymerizations were carried out according to the procedures described in the lab manual1 and the dilute solution viscometry was carried out according to the procedure found on the Macrogalleria.2
Polymerization of PS: In a 10mL Erlenmeyer flask was added 3.67mL of deionized water (continuous phase), 1.8mL of the potassium persulfate free radical initiator, 2.86mL of the sodium lauryl sulfate surfactant, and 2.07mL of the washed styrene monomer. The quasi-emulsion was purged with N2 for about 1 minute and then shaken manually to establish an emulsion. The beaker was placed in an 80°C oil bath (high EA for free radical initiation and propagation) and allowed to stir for one hour. After one hour the emulsion was white. Deionized water was added to the emulsion and then solid NaCl to precipitate out the solid PS. It was then washed with deionized water several times and dried in an oven. The PS was a crumbly white solid.
Polymerization of PEA: In a 10mL Erlenmeyer flask was added 3.67mL of deionized water, 1 drop of ferrous sulfate solution, a tip of a spatula of ammonium sulfate and sodium sulfate, the latter 3 being necessary for doing a redox polymerization, 4 drops of sodium lauryl sulfate surfactant and 2.16mL of ethyl acrylate monomer. This emulsion was purged with N2 for about a minute and then 1.2mL of t-butyl peroxide initiator was added. The emulsion was allowed to stir for 22 minutes and was a milky white when taken off of the stir plate. The polymer was precipitated with solid NaCl, dissolved in about 25 mL of acetone and reprecipitated and washed several times in deionized water. The sample was dried in a vacuum oven overnight and afterwards was clear and gooy (Tg =-24oC)3.
SEC of PS:An SEC sample of PS was made by adding 10mg of PS to 2mL of a THF/toluene solution. The SEC conditions are listed on the data sheet attached at the end of this report.
Dilute Solution Viscometry of PEA: To a 100mL volumetric flask was added 1 g of PEA and the flask was then filled with deionized water. The flask was agitated until the PEA was fully dissolved. The solution was filtered. The efflux time of 7mL of acetone was determined using a Cannon Ubbelohde viscometer in a water bath of 25oC. Subsequently, 2mL of the PEA stock solution were added to the viscometer to make 4 concentrations of the PEA solution and the efflux times of each were determined.
Results and Discussion
The free radical emulsion polymerization of PS and the redox emulsion polymerization of PEA were successful, as polymers were isolated upon the addition of solid NaCl. The surfactant used (for PS and PEA) was charged. Adding salt caused the surfactant to no longer be able to form micelles so the polymers came out of solution. Comparing the two polymerizations is difficult because they were initiated by different types of initiators, at different temperatures and for different amounts of time. In both polymerizations, while the emulsion was being purged with N2, some of the sample bubbled out through the venting needle, which was unavoidable, and could have caused the MW to be lower than what was expected.
The SEC data is attached and reported the following information for PS: Mn=610741g/mole, Mw= 689269g/mole and a PDI (Mw/Mn) of 1.129. Other samples made under the same conditions had Mw's of about 700,000 and 600,000. 4,5 The reported PDI of the PS sample is lower than that of the average emulsion polymerized PS sample. The sample could have somehow been fractionated when cleaned except that the PS was never actually dissolved in anything. It was never clumpy so rinsing it in water got most, if not all, of the NaCl out of the sample and should have not fractionated it since PS is not soluble in water. Even the styrene monomer is only a tiny bit soluble in water so smaller chains of PS would not be soluble at all and thus the PS sample shouldn't have been fractionated just by washing it with water. From data of other students that did this lab it seems that the more an emulsion was stirred before and during the polymerization, the lower the PDI was. The specific sample of this report was shaken before and continuously stirred during the polymerization, which probably afforded the narrow PDI.
The conversion of the monomer was found using the weight of the polymer sample and the amount of initial monomer used. The math is as follows:

The conversions seemed very good but PS was lost in the cleaning process so there must have still been some solvent, monomer or salt still in the sample. The conversion for PEA was considered dependable because none was noticed to be lost while the polymer was cleaned.
The following is the dilute solution viscometry data for PEA in acetone at 25oC:
| [PEA] (g/dL) |
effliux time (sec.) |
avg. time (sec.) |
h/ho | hsp/c | ln(hr)/c |
| 0 | 143.41 | ||||
| (ethyl acrylate) | 143.44 | 143.42 | 1 | ||
| 143.41 | |||||
| 0.222 | 171.84 | ||||
| 171.85 | 171.85 | 1.20 | 0.89 | 0.81 | |
| 171.87 | |||||
| 0.364 | 194.20 | ||||
| 194.22 | 194.09 | 1.35 | 0.97 | 0.83 | |
| 193.85 | |||||
| 0.462 | 207.43 | ||||
| 207.47 | 207.52 | 1.45 | 0.97 | 0.80 | |
| 207.66 | |||||
| 0.533 | 219.94 | ||||
| 219.94 | 220.03 | 1.53 | 1.00 | 0.80 | |
| 220.22 |
Plotting the ln(hr)/c versus concentration and hsp/c versus concentration gives lines with intercepts that should intersect at the intrinsic viscosity of the polymer. The following is such a plot for PEA:
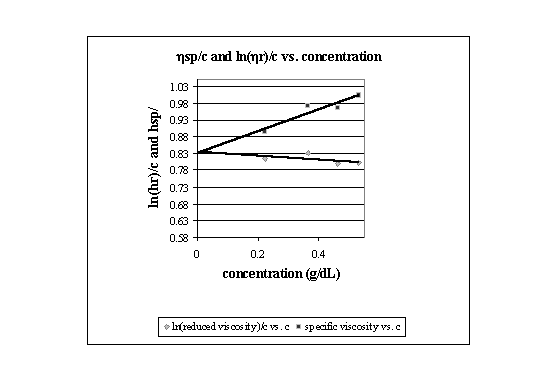
As can be seen by the plot, the intercepts are 0.8349 and 0.8306 dL/g. The average of these numbers was used to find the viscosity average molecular weight of the PEA sample. The Mark-Houwink equation and the K and a values of PEA in acetone at 25oC were used in the following calculations to determine the molecular weight:
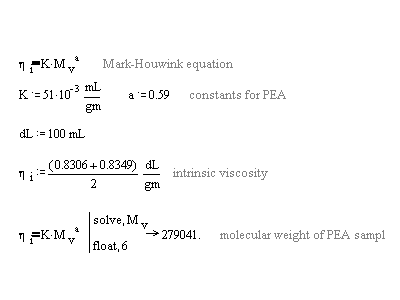
The range of MW that these K and a values can be used in is 300000 to 4500000.3 The sample of this report has a MW outside of that range so the MW is suspect. The molecular weight of this sample of polymer was higher than that of another polymer made with the same amount of initiator by a factor of almost 2 and 3 times larger than a polymer sample made from half the amount of initiator used for the sample of this report.4,6 For emulsion polymerization one would think that a higher initiator concentration would lend to lower MW polymers, however, no clear trend can be seen from the data of other reports on this polymer at the same conditions; some clearly contradicts and some has no correlation to what was expected. Reaction times as well as human error could greatly influence the MW of the samples polymerized. One trend seen by comparing data is that higher MW polymer samples give higher intrinsic viscosities: MW=170030 gives h=0.62 and MW=99000 gives h=0.45.4,6 This trend makes sense because bigger chains (more grams per dL) resist flow more than smaller chains, they can't move as fast so their intrinsic viscosity should be larger.
Conclusions
Free radical emulsion polymerization of PS and redox emulsion polymerization of PEA are both useful and efficient techniques for making PS of low PDI and high MW (~700000g/mole) and for making PEA of moderately high MW (~300000g/mole) using the techniques described above. SEC is an accurate and sensitive way to measure the Mn, Mw and PDI of a polymer sample by fractionating the sample in a chromatographic column. Dilute solution viscometry is a practical way to determine the relationship between MW and intrinsic viscosity for a polymer sample but its accuracy was not checked with respect to the sample analyzed in this report.
References