Poly(vinyl ethers)
Use your mouse on the model to rotate and zoom.
Click items below for other modifications.
spin on spin off
spacefill wire thick wire ball&stick
dots: Vanderwaals dots off
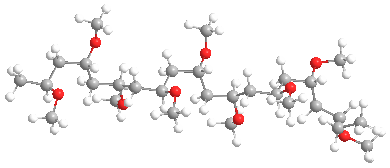
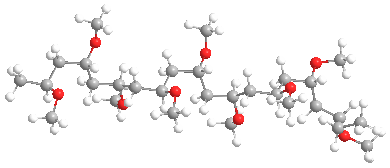
The model above is an image of the methyl vinyl ether polymer.
Click here for 3D model version.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
Poly(vinyl ethers), or PVE for short, are members of a family of low-profile behind-the-scenes polymers with interesting properties. It isn't blatantly obvious where they're found, as is the case with polyethylene or polystyrene. And to be honest, they are structurally similar to poly(vinyl acetate) [aka PVAc] which is the parent of poly(vinyl alcohol) or PVA. The shortest chain versions, such as the methyl ether shown above, are both water and organic solvent soluble. In general, though, they have really lousy properties, mostly being gooey, slimy blobs that are not useful by themselves. We'll talk about that kind first, then recent advances that make available some potentially more useful versions.
PVA is a vinyl polymer, as if you couldn't guess from the name. It's made by free radical vinyl polymerization of the monomer vinyl acetate.
This is what the monomer isobutyl vinyl ether looks like in 3-D:
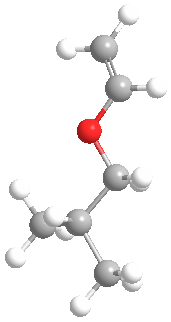
The model above is an image of the vinyl ether you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
One interesting place where PVA likes to hide is in paint cans. So what is PVA doing hiding in your paint? PVA is the latex in acrylic latex paint. So what does that mean? Let me explain. Before PVA can hide in a can of paint, we have to react it with NaOH and methanol like this:
So that leads to the stabilized ionic form of the cation next to a vinyl ether oxygen, one in which one of the oxygen atoms lone pairs is pulled over to form a double bond with the attached carbon. It's not really that simple, but good enough for this discussion. Now everyone's happy, right? Well, happy enough to allow controlled vinyl polymerization but not to give stereoregular polymer. What to do? What to do?
And what about properties? I said that they were all gooey, sticky messes, right? Not actually true for several reasons. First, if you have the right alkyl group attached to the oxygen, you can raise Tg above room temperature. That means it's not gooey anymore at temperatures and pressures we would use the polymer at. And second, you can make it stereoregular, either isotactic or syndiotactic. Or, in fact, you can do both.
Well, then, let's talk about the first method, using the right alkyl group. Turns out adamantyl vinyl ether has been made. Not so hard to envision (structure is given below). And the good news is, despite the bulky adamantane being so near the propagating cation, it undergoes living cationic polymerization quite well. Did I say "living?" You bet1 and that means you can make controlled molecular weight polymer plus block copolymers, if you so desire.
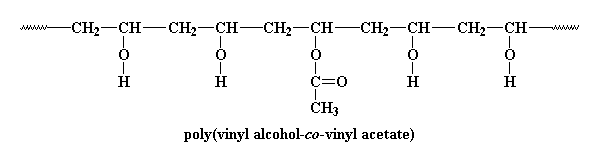
And here's the really interesting fact: the Tg for a 22,000 molecular weight adamantyl polymer is 178 0C! So is the high Tg a result of the "adamantyl effect" or stereoregularity? The answer here is, "Yes!" or "Both," if you want to be picky. The size of the adamantyl group promotes syndiotacticity (or usually does) although I haven't seen NMR proving this one way or the other for this polymer. In any event, the high Tg means this would be a very usable polymer for so many applications.
So what else is new in the vinyl ether business? Well, a report came out recently on using iodide as the counter ion in the vinyl ether cationic polymerization. "Great. So what?" Well, no need to be sassy. What this means is that again the polymerization is living but now with a twist: it's photoactivated. That's right. Turn the light on, propagation; turn it off, it stops. Neat, huh?
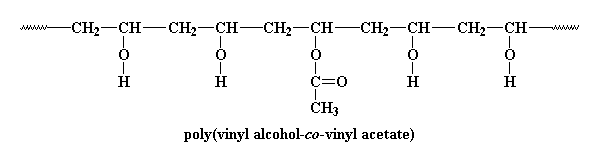
Tested Poly(vinyl ether) Syntheses
Now if you'd actually like to make a poly(vinyl ether), we have two tested methods and one new report for you to look at. Very low temperature cationic polymerization of isobutyl vinyl ether gives highly isotactic polymer while room temperature polymerization proceeds explosively. Be very, very careful if you try that one. Oh, well, nothing fun in life is without some risk and road bumps...Click here to see the procedure and here to download a copy.
NMR Spectra of Poly(vinyl ethers)
We don't have any solution 1H and 13C spectra of any poly(vinyl ether)... yet. But come back later and check; we're looking for them now.We do have solid state spectra of several methyl vinyl ether copolymers with maleic anhydride and maleic acid, plus one solution spectrum of one of these copolymers. You can search the files here to look for spectra you might be interested in.
References
Tamotsu Hashimoto, Yusuke Makino, Michio Urushisaki, Toshikazu Sakaguchi, "Living cationic polymerization of 2‐adamantyl vinyl ether," Vol 46, Issue5, 2008, 1629-1637; https://doi.org/10.1002/pola.22502Nishikawa, Tsuyoshi & Kanazawa, Arihiro & Aoshima, Sadahito; "Metal-Free Photoinitiated Controlled Cationic Polymerization of Isopropyl Vinyl Ether Using Diaryliodonium Salts;" Polymer Chemistry, 10, (2019); 10.1039/C8PY01734D.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |