
How Blueprints are Made
The process of blueprinting goes something like this: A designer or draftsman first makes an original drawing on tracing paper or tracing cloth.
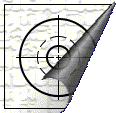
Original Tracing
This tracing is placed over chemically modified blueprinting paper. We'll get to the chemistry of the paper in a moment.
Layover
A bright light is shone on the paper for several minutes.
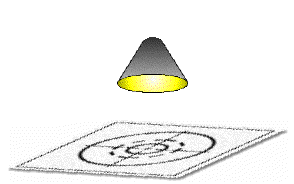
Hit it with Light
The bluprinting paper is then placed in a developing solution where, viola, the paper turns blue, except where the original tracing blocked the light. The bluprint is then washed and dried yielding a perfect negative of the original, give or take a few millimeters after washing and drying.

Finished Blueprint
The Chemistry of Blueprinting
Blueprinting paper isn't just ordinary paper. It's coated with ammonium iron citrate. The formation of this compound is shown below. Amazingly enough, the structure of this inorganic compound isn't known, but we do know that iron is in the +3 oxidation state.
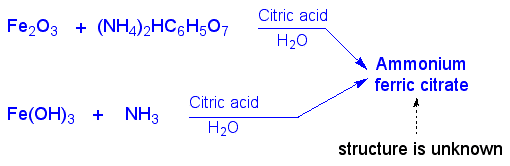
Formation of ammonium iron citrate
Next, the blueprinting paper, with the original drawing on top, is exposed to light. All the iron +3 ions, except those directly under the tracing lines, are reduced to iron +2.
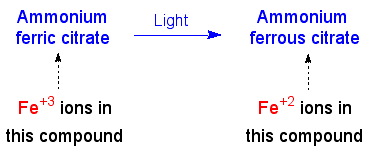
Reduction of Iron +3 to Iron +2
The blueprinting paper, which is still white, is placed in an aqueous solution of potassium ferricyanide. This compound reacts with ammonium ferrous citrate and forms a compound called prussian blue. This compound, in it's hydrated form, is blue. The structure of prussian blue, also known as Turnbull's blue, is still under debate.
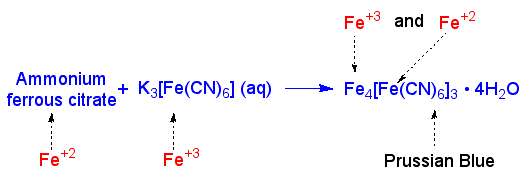
Formation of Prussian Blue
Now deserving of it's name, the blueprint is washed. Prussian blue adheres to the paper while the unreduced iron +2 compound washes away.
Try this experiment but be careful!
Blue-line Prints
Blue-line prints get their names because they have a bluelines on a white background. It sounds almost too simple, doesn't it? These prints are made in the same way as a blueprint, but since they have inverted colors, an original with inverted colors is used. This inverted original is called a brownprint.
The methods described above, though still used, are old and have given way to more
sophisticated techniques.
Whereas blueprint reproductions are negatives of an original drawing, the diazo
(whiteprinting) process provides positive images like those of blue-line prints,
except the lines can be of almost any color on a white background.
The paper is coated with a diazo compound that is sensitive to
ultraviolet light. Just as in blueprinting, the lines of the original protect
areas beneath it. Whiteprints are developed in an ammonia atmosphere, but in this
case, the unexposed areas, the lines in this case, change color.
So you're scratching your head and wondering what all this has to do with polymers, huh?
Well, to start, paper is made of polymers, and. . . Aw heck, you caught us. We weren't
trying to pull a fast one, honest. The topic just seemed so darn interesting. And if
you're reading this far, perhaps you thought so too. Where're the polymers?
But to be honest, the PAPER that all
this takes place on has polymers in it... click on that link to see more.
![]()
Return to How Polymers Work