anion
cation
living polymerization
covalent bond
termination
Anionic vinyl polymerization is a method of making polymers from small molecules containing carbon-carbon double bonds. It is a type of vinyl polymerization. In anionic polymerization, the process is begun by an initiator. In this case, the initiator is an anion; that is, an ion with a negative electrical charge.
There are a lot of different initiators used in anionic vinyl polymerization, but the most often used is an unassuming little molecule called butyl lithium.
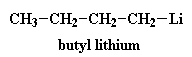
Now a little bit of the butyl lithium will always fall apart. Not a lot, but some. It falls apart to form a positive lithium cation and a negative butyl anion. We call an anion like this where the negative charge is on a carbon atom a carbanion
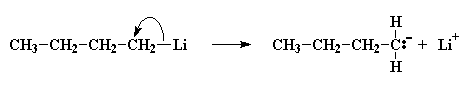
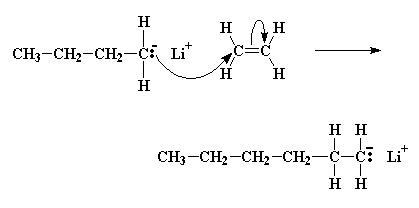
A pair of electrons from the butyl anion will be donated to one of the double bond carbon atoms of the monomer. Now this carbon atom already has eight electrons in its outer shell which it shares with the atoms to which it is bonded, so one pair of these electrons, specifically a pair in the carbon-carbon double bond, will leave the carbon atom, and settle on the other carbon atom of the carbon-carbon double bond. This forms a new carbanion, with the negative charge resting on that carbon. The process in which the butyl lithium falls apart, and the butyl anion reacts with a monomer molecule is called initiation.
Click here to see a movie of the initiation reactions.
The carbanion now reacts with another monomer molecule in just the same manner as the initiator reacted with the first monomer molecule; another carbanion is generated. This keeps happening, and each time another monomer is added to the growing chain, a new anion is generated allowing another monomer to be added. In this way the polymer chain grows. This adding of monomer after monomer is called propagation.
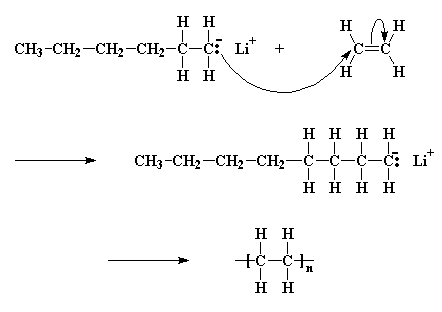
Click here to see a movie of the propagation reactions.
The Chain That Wouldn't Die
Now while you may get this nagging feeling that this can't go on forever, that something has to put a stop to this convenient process. Funny thing, though: it doesn't stop! In many cases, the only thing that stops monomers from adding to the growing chain, is that eventually there are no more monomer molecules in the beaker left to add! And even then, if someone came along some time later and dumped more monomer into the beaker, they would add to the chain and the chain would grow some more! Some chains of polystyrene have been known to stay active like this for years. In order to stop them, something like water, which reacts with the carbanions, has to be added to the polymer. Systems like this are called living anionic polymerizations. This allows us to do some interesting tricks…
Modular Chemistry
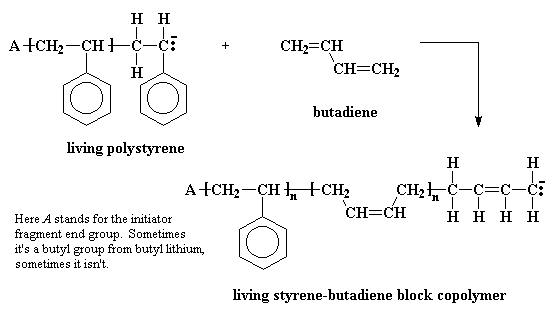
Some time ago someone was pondering this beaker full of polymer which would stay active for years; and how if one added additional monomer, this new monomer would add to the living polymer chains, and came up with an idea. Instead of adding the same monomer to the solution filled with living polymer, why not add a different monomer? The result was a polymer whose chains consisted of a long stretch of one type of polymer, and a second long stretch of another polymer. Polymers like this are called block copolymers. For example, a solution of living polystyrene chains will react with butadiene to give a styrene-butadiene block copolymer.
A few more tricks will give us a styrene-butadiene-styrene triblock copolymer. Don't you wish you knew how we do this? To find out click here.


|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |