Keywords
living polymerization
termination
So you want to know how we make SBS rubber. Being the nice people you are, we're going to tell you. It goes something like this: We start off using a technique called living anionic polymerization. A living polymerization is a polymerization that takes place without any termination reactions. This means that once all the monomer in your beaker is used up, and has been turned into polymer, the polymer chain ends are still active. This means that if you put more monomer in the beaker, it would add to the polymer ends and make the polymers bigger. This lets us do some tricks.
Wanna see what they are?
The first thing we have to do is make a chain of living polystyrene. This is done by polymerizing the monomer styrene with an anionic initiator like butyl lithium.
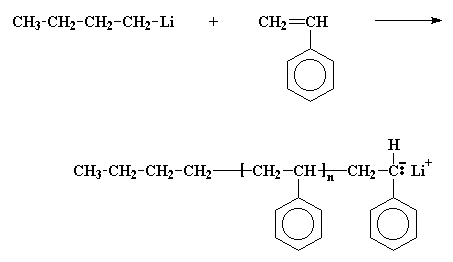
Remember now, this polystyrene chain is living, so if we add a second monomer to it, it'll add to the polymer. So we'll add some of the monomer butadiene.
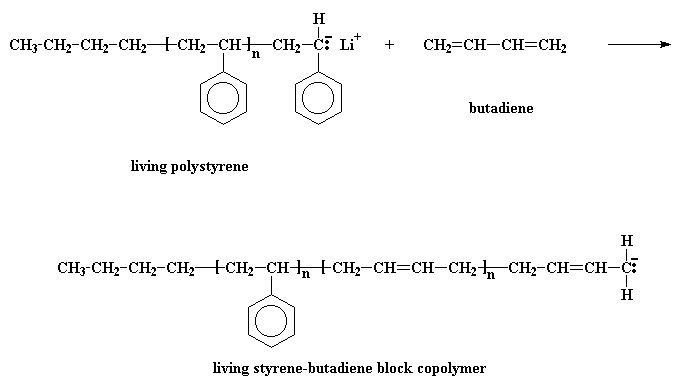
That gives us a living styrene-butadiene block copolymer.
The next step is obvious: just add more styrene monomer, and get a styrene-butadiene-styrene triblock copolymer. Easy as pie. Funny thing, though. Although butadiene monomer will add to the anion at the end of a polystyrene chain, styrene monomer won't add to the anion at the end of a living polybutadiene chain. This is most inconvenient. To get around this, we do a little trick: we're going to react it with a compound called dichlorodimethylsilane.
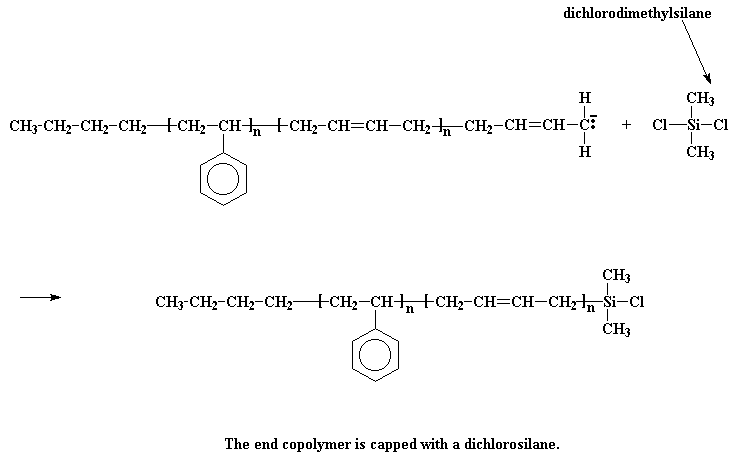
The anionic living chain kicks off a chlorine atom from the silane, and we get a chlorosilane end-capped polymer. So what good is that? Now our polymer is no longer living! It's good because we can do something with this chlorosilane end-capped polymer. You see, if we take living polystyrene homopolymer, it will react with the chlorosilane end-capped polymer, just like the styrene-butadiene copolymer reacted with the dichlorodimethylsilane.
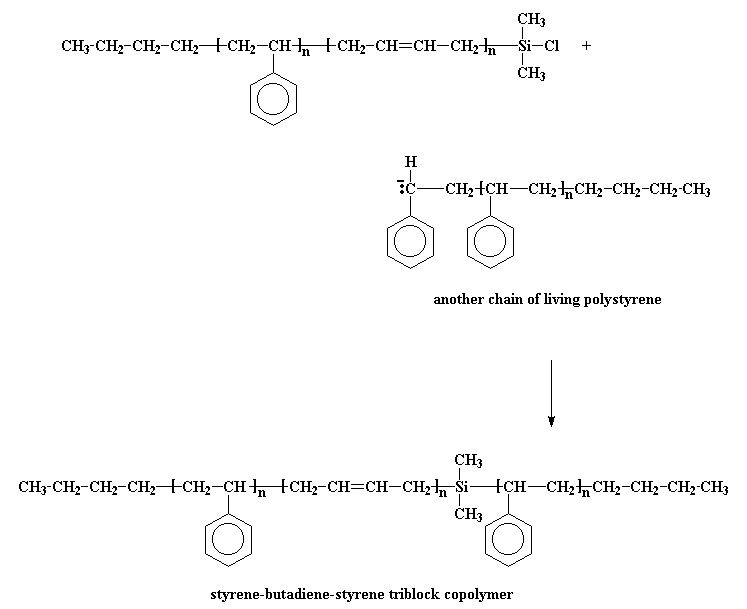
This gives us the triblock copolymer we wanted. Right nifty, if you ask me.
Oh, so you want to know why we didn't just add more of the first monomer, styrene to the living diblock chain instead of the silane terminating group? Great questions! And the answer is: that works, too. And that method is used with other monomers as well.
You could add a third (different) monomer to the living diblock polymer before adding the silane, and that would have given you a triblock with each block being different. People (scientists, I'm sure you guessed) have done this ad nauseum and made polymers with five, seven and even more different or the same blocks in all kinds of combinations and chain segment lengths.
This kind of anionic polymerization gives you tremendous capability to make multi-block copolymers. The only stipulation should be obvious: all the monomers must be capable of anionic polymerization. Can you think of any way around that requirement? Scientists have, but we'll leave you wondering for now...

|
Return to Anionic Polymerization Page |

|
Return to SBS Rubber Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
