anion
cation
complex
covalent bond
ion
Cationic vinyl polymerization is a way of making polymers from small molecules, or monomers, which contain carbon-carbon double bonds. Its primary commercial use is for making polyisobutylene.
In cationic vinyl polymerization, the initiator is a cation, which is an ion with a positive electrical charge. It is shown as A+ in the picture. A pair of electrons, negatively charged, from the carbon-carbon double bond will be attracted to this cation, and will leave the carbon-carbon double bond to form a single bond with the initiator, as shown. This leaves one of the former double bond carbons at a loss for electrons, and carrying a positive charge. This new cation will react with a second monomer molecule in the same manner as the initiator reacted with the first monomer molecule. This happens over and over until a high molecular weight is reached, that is, a molecular weight at which the polymer is useful for something.
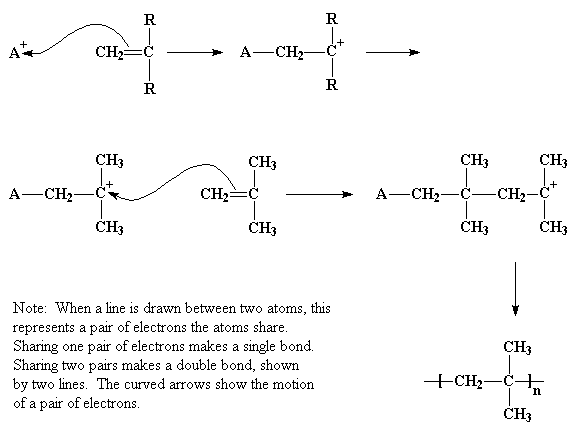
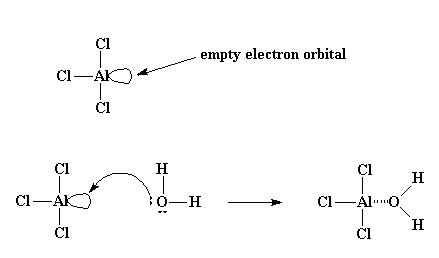
Many times, though, it starts off in little bit more complicated manner than that. Normally, the initiator used is something like aluminum trichloride, or AlCl3. If you know the octet rule, you'll know that all atoms on the second row of the periodic table like to have eight electrons in their outermost shell, or level. The aluminum atom in AlCl3 is sharing electron pairs with only three other atoms, leaving it with only six electrons, two short of the magical octet. As it sits, it has a whole orbital (that is, a vacant slot where a pair of electrons should be) empty and ready for something to come along and fill it. It just so happens, much to the delight of that aluminum atom, that a very small amount of water is usually present in the system. Now the oxygen atom in water has two unshared pairs of electrons, and it most graciously donates a pair to the aluminum atom, forming an AlCl3 and H2O complex.
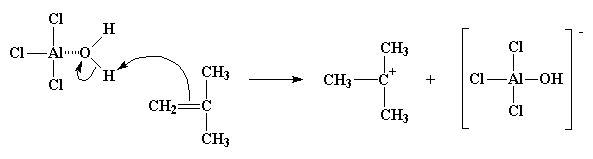
Oxygen, being very electronegative, will tend to pull the electrons it shares with the hydrogens atoms toward itself, leaving the hydrogen atoms with a slight positive charge. This leaves them ripe for attack by a pair of electrons from the double bond of a monomer molecule. The monomer in this way can swipe the hydrogen, making itself a cation, and the AlCl3/H2O complex becomes its compliment anion, AlCl3OH-. This whole process by which the AlCl3/H2O complex forms and reacts with the first monomer molecule is called initiation.
You can see we've got a new cation after all this is done. Not just a cation, a carbocation. That's what we call a cation where the positive charge is on a carbon atom (duh!). Carbocations are very unstable. They're unstable because the carbon atom in a carbocation only has six electrons in its outermost shell. Six! That's two electrons short of the eight that all carbon atoms want to have in their outermost shells. So a carbocation will do just about anything to get two more electrons and reach the magic octet.
So the carbocation looks around, and finds a pair of electrons in the double bond of a nearby monomer molecule. (Remember there are two pairs of electrons in a double bond.) So the carbocation swipes those electrons, and in doing so forms a single bond with the monomer molecule. It also generates another carbocation, as you can see in the picture below. This can react with another monomer, and then another, and so on. Eventually we get a long polymer chain.
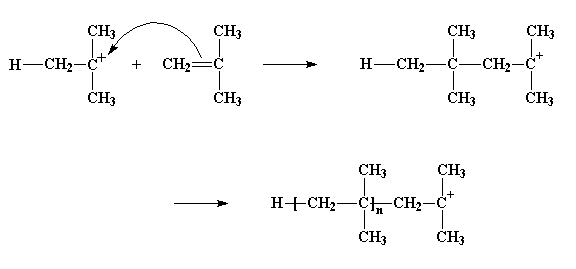
This process, by which monomer after monomer is added to form a polymer, is called propagation.
But how does it end? How does this cycle of adding more monomer molecules to the growing polymer stop? The most common way goes something like this... Imagine the growing chain of polyisobutylene. The methyl groups attached to the cationic carbon atom have a little problem: they've got hydrogen trouble. The hydrogens on these methyl groups will, without a whole lot of persuasion, break away and join other molecules. This is just what can happen when they come close to a molecule of monomeric isobutylene. Starved for electrons as they are, being part of a cation, these hydrogens are easily attacked by a pair of electrons from the carbon-carbon bond in the isobutylene molecule. When all the electrons are through rearranging themselves, as shown by the arrows, we're left with a neutral polymer chain with a double bond at its end, and a new cation, formed from the isobutylene molecule. The polymer chain end is now neutral, and can no longer react and grow. But the new cation can start a new chain growing, in the same way as our initiator molecule did. This process is called chain transfer. It also happens in free radical polymerization, and other kinds of polymerization as well.
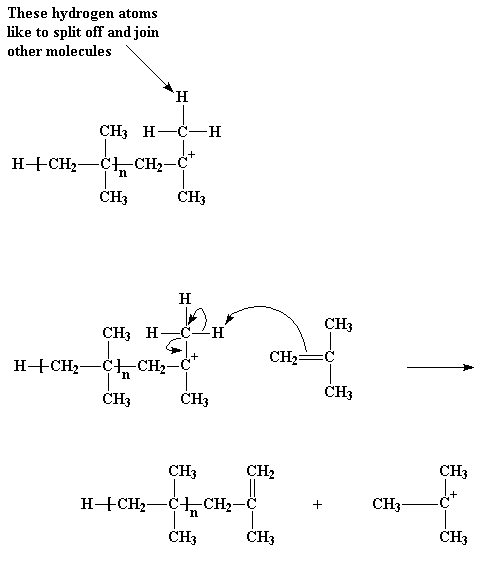
That particular kind of chain transfer is called chain transfer to monomer. But there's another kind of chain transfer. To understand, it helps to remember that for every cation, there is an anion lurking somewhere in the same beaker. Remember that AlCl3OH- ion? As we all know, cations and anions have this nasty tendency to react with each another, which can be troublesome when we want our cation to react with something else, like a monomer molecule. Let's take a look at how this happens.
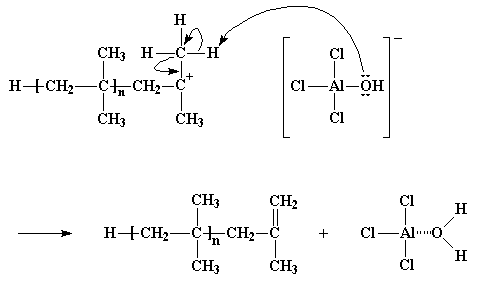
When the cationic initiator reacts, and forms a growing cationic chain, the old anion of the initiating cation becomes the anion of the growing polymeric cation. Remember that anions tend to have pairs of electrons floating around with nothing to do, and, what did mother always say, idle hands do the devil's work? That's just what happens here. The electrons of the anion will, from time to time, attack the hydrogen atoms on the methyl groups adjacent to the cationic carbon. Remember these hydrogen atoms? They're the ones that were so eager to be snatched away by a nearby monomer molecule. Sometimes, not often but sometimes, these hydrogens will very easily react with the unshared electrons of the oxygen atom in the anion in the exact same manner, leaving the same dead polymer chain with a double bond at its end. But on the bright side, the AlCl3/H2O complex is regenerated, and it can start new polymers growing just as it did earlier. Yet another case of the villainous chain transfer.
Of course, it doesn't always turn out so well. At times it is the unshared pairs of electrons from a chlorine atom which will react, not with one of the hydrogen atoms, but with the cationic carbon atom itself. It leaves the counterion, and joins the polymer. We're then left with a different kind of dead polymer, ending in a chlorine atom, and AlCl2OH, which won't start a new polymer chain growing.
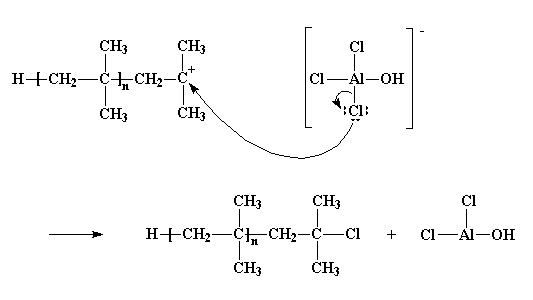
This process is called termination, because no new chains are started. It's the last of the three major steps in any chain growth polymerization, the first two being initiation and propagation, of course. When termination happens, the polymerization is over.
Ok, we've spent all this time basically talking about just one monomer that undergoes cationic polymerization: PIB. There are others, such as vinyl ethers. In fact, while vinyl ethers have been made in the past by free radical polymerization and an uncontrolled version of cationic polymerization, recent advances have made this family of fascinating polymers available in more uniform compositions, in higher yields, in higher molecular weight, in block copolymers, and most interesting, in the stereoregular isotactic form. And if that doesn't whet your whistle to go look at poly(vinyl ethers) in more depth, then try one of the other links on the left.


|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |