Most likely you're sitting in a chair right now. This chair probably has a cushion to make it more comfortable. And that cushion is most likely made from a polyurethane foam. So are the cushions of the seats in your car, and probably the pillows you sleep on, as well as the cushions of your couch. That's right, couch potatoes, your relaxed lifestyle depends on the existence of polyurethane foams.

The most important use of
polyurethane foams.
"But how do we make these all-important foams?" you ask. The simple answer is, "add a touch of water when you make the polyurethane". It's as simple as that. We'll show you.
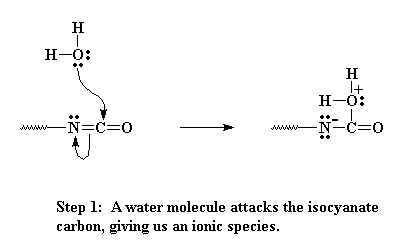
Remember from the page on making polyurethanes where we talked about oligomers, dimers, trimers, on up to polymers? Then you know that those oligomers can have isocyanate groups on the chain ends. That carbon in the isocyanate group is sandwiched right between two electronegative atoms, oxygen and nitrogen (see the figure below). Remember electronegative atoms will pull electrons away from other atoms. So the nitrogen and the oxygen leave that poor carbon really hungry for some electrons. Now let's add that water we talked about. Each oxygen has two lone pairs of electrons which can be attracted to and react with that partially charged carbon of the isocyanate.
And don't forget base catalysis. A tertiary amine base, for example, will polarize the water O-H bond even more, sometimes to the point of total ionization (and I didn't say annihilation). The gives an H-O- anion which is even more nucleophilic than just plain old water.
Click here to see a movie of this reaction.
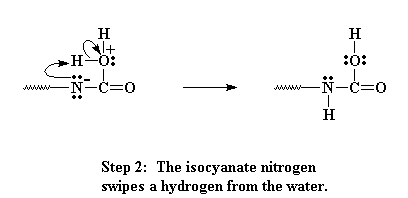
Now the oxygen and carbon form a new bond, liberating on pair of electrons that can be pulled back onto the nitrogen as a negative charge. Nitrogens don't like having a negative charge like that, so it looks for a way to balance the charge. It does so by swiping a hydrogen from the bound water attached to the carbon.
Click here to see a movie of this reaction.
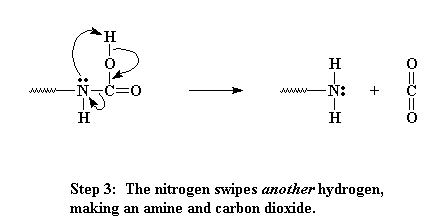
Never satisfied, that nitrogen then decides to swipe another hydrogen from the same place. When this happens, carbon dioxide gas is expelled and we end up with an amine on the end of our oligomer.
Click here to see a movie of this reaction.
If you want to see a movie of the whole process of water attacking the isocyanate to form the amine, just click here you will surely see it.
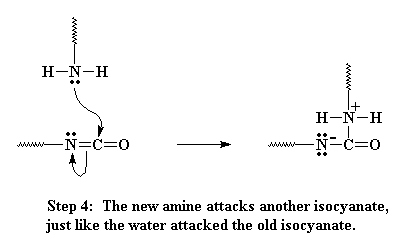
This amine can react with another isocyanate group, just like the water did, only this time, we end up with these two oligomers joining, and they are joined by a urea linkage when its all over.
Click here to see a movie of this reaction.
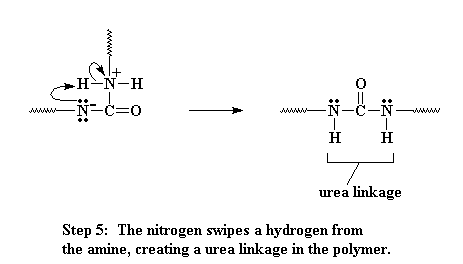
Click here to see a movie of this reaction.
If you want to see a movie of the whole process of the amine reacting with the isocyanate to form the urea, click here.
So big deal. Our polyurethane has a few urea linkages instead of urethane linkages. Who cares? Well, to be honest, nobody does. The urea linkage doesn't alter the properties of the polymer that much. What is important is what happened back in step three. Remember that carbon dioxide gas that was expelled? This gas is very important.
Here's why:
When the polymerization reaction begins, the mixture is a liquid, and any carbon dioxide that is produced just bubbles away. No problem. But as the reaction progresses, and molecular weight increases, the mixture will become more and more viscous. As this happens, the carbon dioxide bubbles will be trapped in the viscous liquid. When the polymer finally solidifies, they stay there, trapped. And it's these bubbles that make the polymer a foam!

|
Return to the Making Polyurethane Page |

|
Return to the Polyurethane Page |

|
Return to Macrogalleria Directory |
