This is a most exciting, page, really! This is the page where you
will learn how to make polyurethanes! And it's not
all that hard. We'll prove it to you. Read on...
To start off, we make polyurethanes from two monomers, a diol and a diisocyanate. There's a huge variation in the structures available for both types of monomers. Many of these are actually used to make polymers for a wide range of applications, from hard varnishes and furniture coatings to soft flexible foam cushions. But here we'll look at just a few examples, such as two of the simplest monomers shown below:
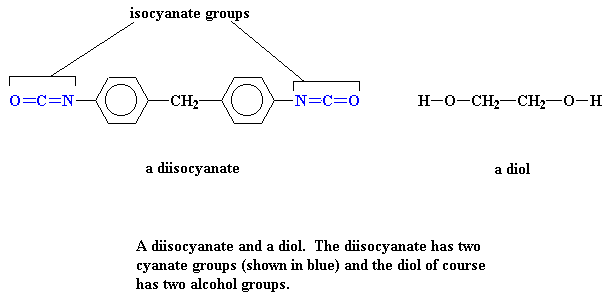
You can see two these monomers in 3-D by clicking on their images.
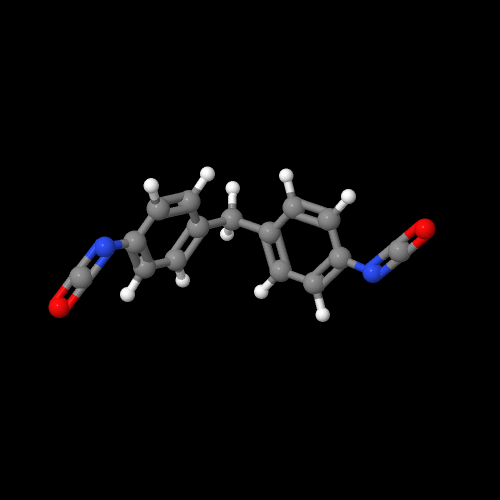
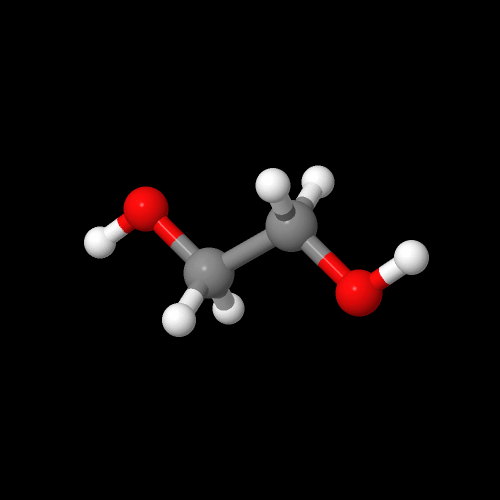
Be sure to close the new window that opens up when you are ready to come back here.
Basic Catalyst Mechanism
With the help of a little molecule called diazobicyclo[2.2.2]octane, or DABCO for short, we can make these two polymerize. When we stir the two monomers together with DABCO, something nifty happens.
DABCO is a very good nucleophile. That is, it has a pair of unshared electrons on each of the nitrogens that would just love to attach themselves to a positively charged nucleus. Remember, electrons have negative charges, and the nuclei of atoms have positive charges. And we all know that negative charges and positive charges attract.
So DABCO's electrons look around, and they find a nucleus in the form of the alcohol hydrogens of the diol. These hydrogens are vulnerable, because they are weakly bonded to oxygen atoms. Oxygen is electronegative. This is to say it pulls electrons away from other atoms. So it pulls electrons away from its neighbor the hydrogen atom. This leaves the partial positive charge on the hydrogen unbalanced. In other words, the hydrogens have a slight positive charge.
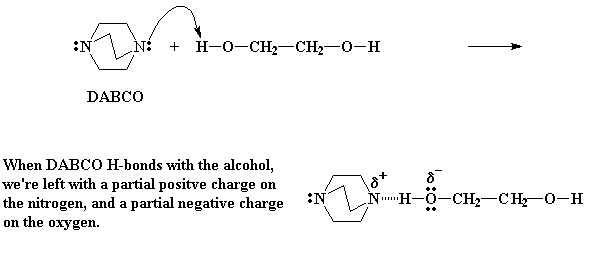
Click here to see a movie of this reaction.
When DABCO's electrons see this and they just can't help themselves. They rush over and form a hydrogen bond between the hydrogen and DABCO's nitrogen. This H-bonding leaves a partial positive charge on the nitrogen, but more importantly, a partial negative charge on the oxygen. This partial negative charge makes the oxygen really hot. Being hot as it is, it wants to react with something.
Would you like to know just what it will react with?
The oxygen has an excess of electrons, so it will react with something that is poor in electrons. If we look at our isocyanate, we can see that the carbon in the isocyanate group is sandwiched between two electronegative elements, oxygen and nitrogen. This means that this carbon is going to be very poor in electrons indeed. So our hot oxygen wastes no time in reacting with it. It throws a pair of electrons to that carbon, and a bond forms.
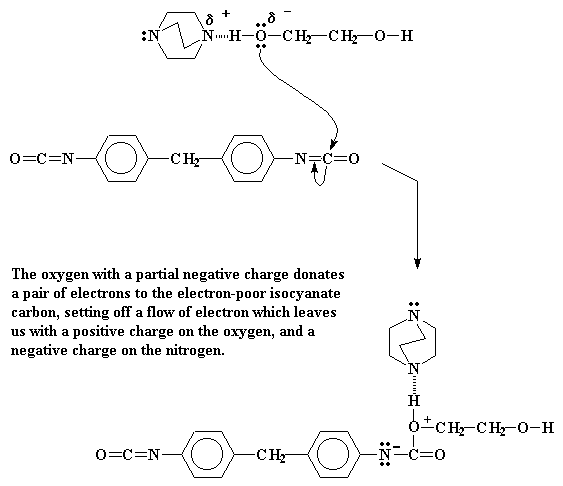
Click here to see a movie of this reaction.
Of course this pushes a pair of electrons out of the carbon-nitrogen double bond. This pair situates itself on the nitrogen, giving it a negative charge. Our oxygen meanwhile, having donated an electron pair, is left with a positive charge.
Now there isn't much that a nitrogen atom likes less than to have a negative charge. So it's going to try to get rid of it as soon as it can. The easiest way to do this is to donate that pair to our old friend, the alcohol hydrogen atom. This forms a bond between that hydrogen and the nitrogen.
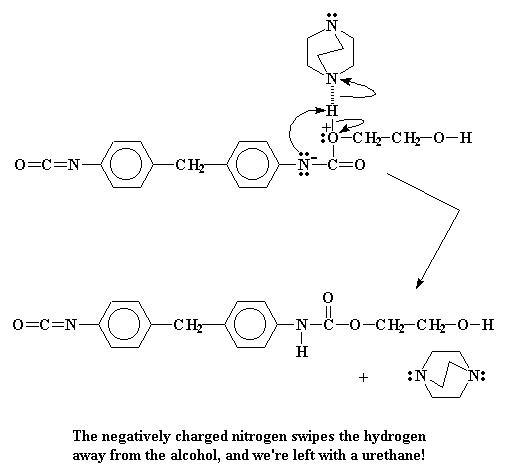
The electrons that the hydrogen had shared with the oxygen now belong to the oxygen alone. This eliminates that old positive charge that the oxygen was carrying. When it's all over we're left with a brand new urethane dimer.
This urethane dimer has an alcohol group on one end, and an isocyanate group on the other, so it can react with either a diol or a diisocyanate to form a trimer. Or it can react with another dimer, or a trimer, or even a higher oligomers. In this way, monomers and oligomers combine and combine until we get a high molecular weight polyurethane.
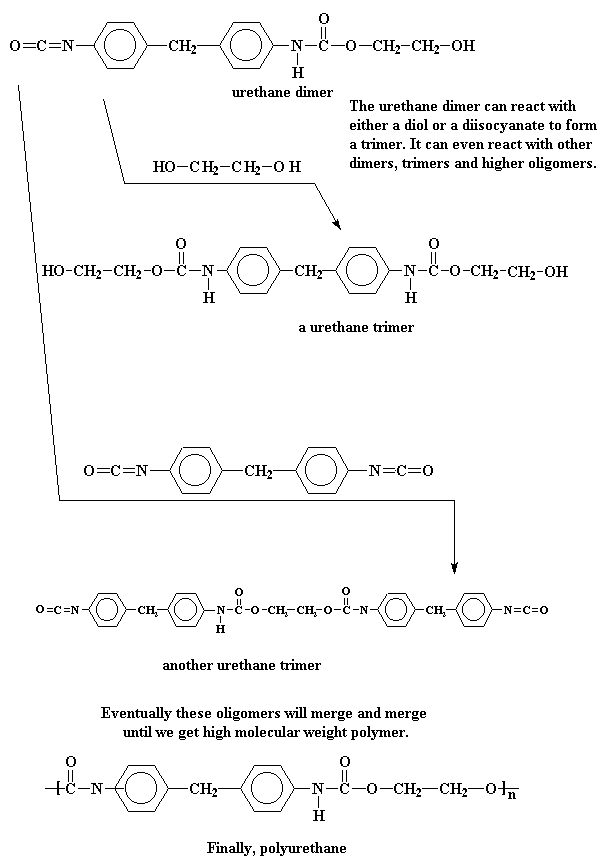
For those of you keeping score at home, you'll notice that not only monomers react, but also dimers, trimers, and so on. This makes it a step growth polymerization. Also, because no small molecule by-products are produced, it is an addition polymerization.
Polymers Within Polymers
Sometimes, instead of using a small diol like ethylene glycol, we use a polyglycol, one with a molecular weight of about 2000.
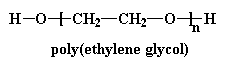
This gives us a polymer within a polymer so to speak, and we have a polyurethane that looks something like this:
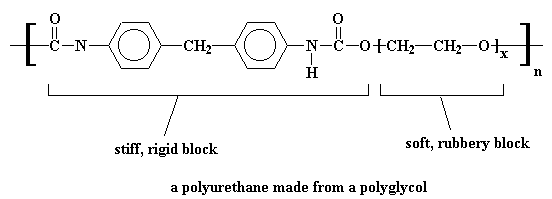
If you like, you can learn how to make a polyurethane foams.
And for those of you who are really curious about catalysis and why DABCO is so good at it, you can see DABCO in 3-D by clicking on its image below.
Be sure to close the new window that opens up when you are ready to come back here.

|
Return to the Polyurethane Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
