
Some Polymer Basics
This is sort of an introductory page for a more in-depth look at polymers. The first issue to get out of the way is this: when we speak of polymers, just what exactly are we talking about? What is a polymer and what isn't? Normally the word polymer is used when talking about molecules whose molecular weight (or size) is in the range of several thousand or more. More or less. Pretty simple, huh? Sure, but as people say nowadays, "It's complicated." While it sounds simple, polymers are, in fact, very different from smaller molecules.
Get in Line!
But usually we like to be more picky than that. Most of the time when we talk of polymers we're talking about molecules with molecular weights of hundreds of thousands, or even millions. We're also usually talking about linear polymers.
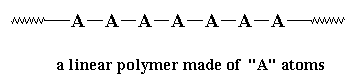
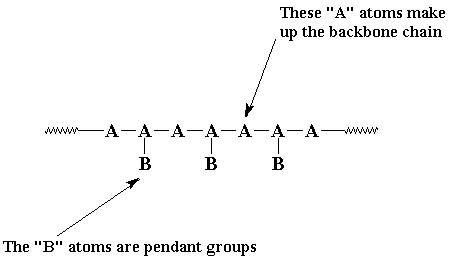
A linear polymer is a polymer molecule in which the atoms are more or less arranged in a long chain. This chain is called the backbone. Normally, some of these atoms in the chain will have small chains of atoms attached to them. These small chains are called pendant groups. The chains of pendant groups are much smaller than the backbone chain. Pendant chains normally have just a few atoms, but the backbone chain usually has hundreds of thousands of atoms.
Polymers Are Like TV: Both Have Lots and Lots of Repeats
Normally, too, when we talk of polymers, we're not just talking about huge molecules whose atoms are arranged in chains. We like to think that the atoms that make up the backbone of a polymer chain come in a regular order, and this order repeats itself all along the length of the polymer chain. For example, in polypropylene, the backbone chain is made up of just two carbon atoms repeated over and over again. One carbon atom has two hydrogen atoms attached to it, and the other has one hydrogen atom and one pendant methyl group.This unit of a carbon atom with two hydrogen atoms followed by a carbon atom with a hydrogen atom and a methyl group repeats itself over and over again along the backbone chain. This little recurring structure is called the repeat structure or the repeat unit.
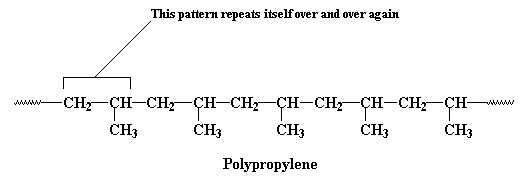
To make things simple, we usually only draw one unit of the repeat structure, like this:
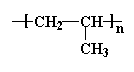
The repeat unit is put inside brackets, and the subscript n just stands for the number of repeat units in the polymer chain. That "n" can be anything from a few hundred to several million. We'll find out, though, that polymer chains with millions of units are pretty much useless. They're so big and so entangled that they won't easily mold or extrude into fibers.
Breaking the Line
Polymers can come in other structures, though. To find out, take a look at the nonlinear polymer page.
The Consequences of Being Big
Let's get back to those simple linear polymers, now. These giant chain-like molecules, because they are so big and because of their shape, act in ways which small molecules don't. There are three reasons for this. To find out what they are, take a look at the page we call Three Things That Make Polymers Different.
Some Assembly Required
Polymers don't start out big. They start as little tiny molecules called monomers. To make a polymer, a whole mess of monomers are strung together in a line to form a long polymer chain. For example, styrene monomers are joined together to make polystyrene:
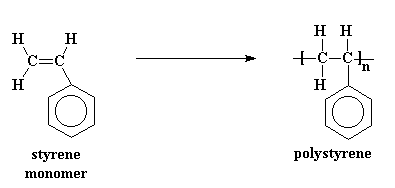
For more on building polymers from monomers, go here.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |