gem diol
ring-opening polymerization
Nylon 6 is an awful lot like our friend nylon 6,6. You can look at the picture if you don't believe me.
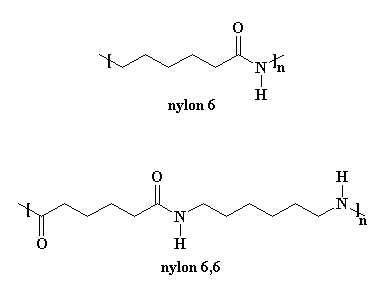
But making nylon 6 is lot different from nylon 6,6. First of all, nylon 6 is only made from one kind of monomer, a monomer called caprolactam. Nylon 6,6 is made from two monomers, adipoyl chloride and hexamethylene diamine.
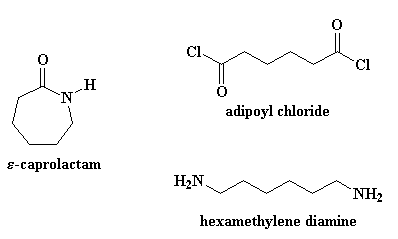
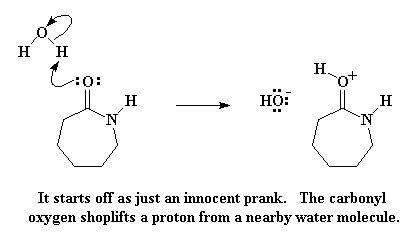
But enough small talk. Let's get on with the business of turning caprolactam into nylon 6. Nylon 6 is made by heating caprolactam to about 250 oC with about 5-10% water thrown in. So what happens to caprolactam when there's water around? The carbonyl oxygen looks around, and sees a water molecule, and sees how easy it would be to steal one of the water's hydrogen atoms. Now as is often the case, a little thing like this that seem harmless enough can grow into something much bigger. If you watch, you'll see that caprolactam's greed is going to get the better of it.
Click here to see a movie of this reaction.
The carbonyl oxygen donates a pair of electrons to the hydrogen atom of water, thus stealing the hydrogen from the water. This gives us a protonated carbonyl, and a free hydroxyl group. Keep this hydroxyl group in mind, because it is going to come back to haunt greedy ol' caprolactam. But first, let's remember that the carbonyl oxygen now has a positive charge. It doesn't like this, so it swipes a pair of electrons from the carbonyl double bond, leaving the positive charge on the carbonyl carbon atom.
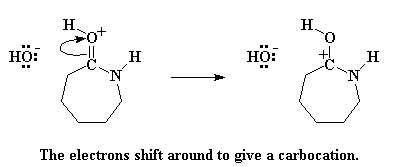
But carbocations are not happy critters. Putting a carbocation in a molecule is just begging for some nucleophile to come along and attack it. Nucleophile? Did someone say nucleophile? I think there's one nearby. It's that old hydroxide ion that was left when caprolactam stole the proton from the water molecule. This little hydroxide ion never really worked through the negative emotions of having lost its proton to caprolactam. Still harboring a lot of hostility, it attacks the carbocation.
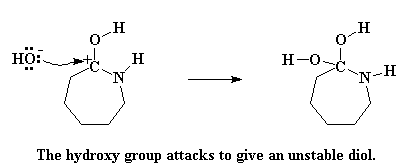
Click here to see a movie of this reaction.
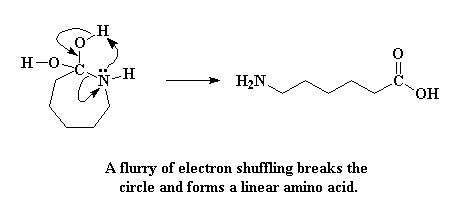
The molecule formed is now an unstable gem diol. Unstable? Of course. Didn't I tell you that caprolactam's greed would be its undoing? A mad reshuffling of electrons happens next. The nitrogen atom donates a pair of electrons to a hydrogen atom on one of the hydroxyl groups, stealing it away. The electrons that the hydrogen shared with its oxygen shift to form a double bond between the oxygen and the carbon atom. And lastly, the electrons shared by the carbon and the nitrogen shift completely to the nitrogen, severing the carbon-nitrogen bond.
Click here to see a movie of this reaction.
Alas, the circle is broken, and caprolactam is no more! Like many junk bond dealers in the eighties, it has paid dearly for its greed. What we're left with is a linear amino acid.
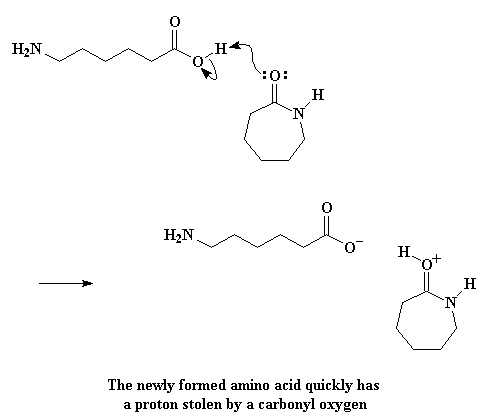
But our story is far from over. You see, that linear amino acid can react with a caprolactam molecule, a lot like the water molecule did. Caprolactam molecules aren't very bright. Witnessing one of their own destroyed by greed doesn't make them any less greedy. They just try to steal what they can from their fallen sibling, like greedy little buzzards. Ever avaricious, a caprolactam molecule will steal the acid hydrogen form the linear amino acid. The carbonyl oxygen donates a pair of electrons to that hydrogen, stealing it away from the amino acid.
Click here to see a movie of this reaction.
And as expected, the electrons rearrange to form the carbocation, just as before:
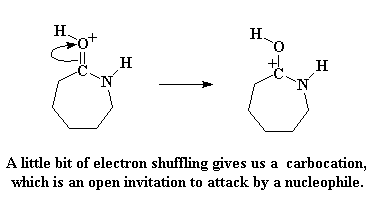
This carbocation is still an open invitation to any nucleophile around, but this time, there's a new nucleophile on the block. That's the amino acid that just lost its acid hydrogen. It too has a lot of hostility towards the thieving caprolactam, and attacks just like we saw the hydroxide ion attack earlier.
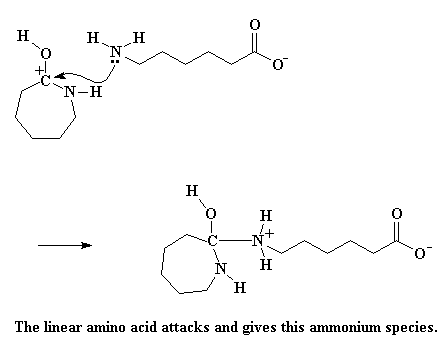
Click here to see a movie of this reaction.
This gives us an ammonium species, and this particular one is very unstable. The electrons play musical chairs. Showing no elemental loyalty, the ring nitrogen steals a hydrogen from the ammonium nitrogen. In addition, the bond joining the carbon and the nitrogen is severed, opening the ring. Another greedy caprolactam molecule bites the dust.
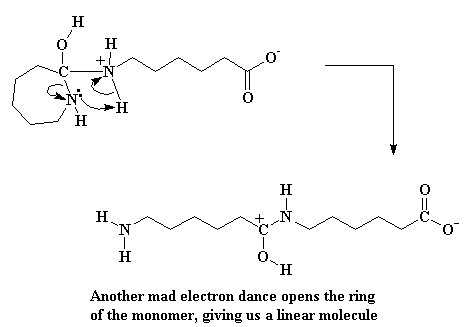
Click here to see a movie of this reaction.
But we're not through yet. That carboxylate group at the end of the molecule is going to sweep around and steal the alcohol hydrogen.
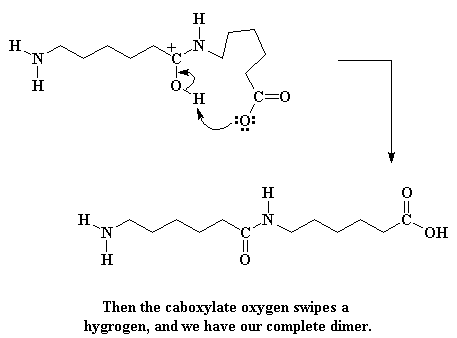
Click here to see a movie of this reaction.
This makes a new carbonyl group in the middle of the molecule, and regenerates the carboxylic acid. (Here's a little secret: No one really knows the order of the last two steps. They might happen in the reverse order. We just know that both of them happen before everything is over.) If you want to see a movie of the whole propagation process, click here. (Remember, this is a step growth polymerization.)
Now that we have an acid again, it is sure to react with another avaricious caprolactam molecule, and then another, and another, until we get long chains of nylon 6.
Ok, so that's how nylon 6 is made industrially (as far as we know) but it's not all that easy to make it in the laboratory this way. Besides, the step-growth mechanism above has some of the characteristics you already know about, right? If you forgot, you might want to review by going here.
Tested Nylon 6 Synthesis
To make a nylon 6 sample the way it's made industrially (or close to it), we have a procedure for you to follow. It uses caprolactam, water and diacid-diamine salt as catalyst/initiator. An interesting feature of this method is that the initiator makes a two-armed star (if you can call it that) with either one each or two each acid and/or amine end-group.This method is simple and easy, so click here to view the procedure and here to download a copy.
Nylon 6 NMR Spectra
Let's assume you have a sample of nylon that you're pretty sure is nylon 6. How do you confirm that hypothesis? Easy! Take an NMR spectrum or two. But wait! You need a standard to compare it to, right? Have we got a deal for you: both proton and carbon spectrum of commercial nylon 6.
Go here to view a 1H spectrum and and here to see the 13C spectrum.
Can We Make Nylon 6 Some Better Way?
So two of the key characteristics of chain-growth polymerization are using an initiator that controls one of the two chain end functional groups, and being much faster than step-growth (in general anyway). Another characteristic we haven't much talked about is molecular weight. With step-growth, you run into a concentration problem: near the end of functional group conversion, there just isn't a very high concentration of chain ends to find each other. That means the propagation basically grinds to a halt and molecular weight is middlin' at best. While that's fine for PET and most nylons for commercial uses, won't it be nice to make some really high molecular weight material? Why not use a chain-growth mechanism to do that?
Great suggestion! And it works really well for nylon 6. Let's think about it a little... Ok, enough contemplation. Go to the section here to find a more in-depth explanation of chain-growth synthesis of nylon 6 by anionic polyaddition.

|
Return to Nylon Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
