Le Chatelier's principle
oligomer
This page is all about how to make nylon. We're telling you this as if you can't read the title for yourself. To start off, nylon is made by a reaction which is a step-growth polymerization, and a condensation polymerization. Nylons are made from diacids and diamines. If you want to see what adipic acid and hexamethylene diamine look like in 3-D, click here.
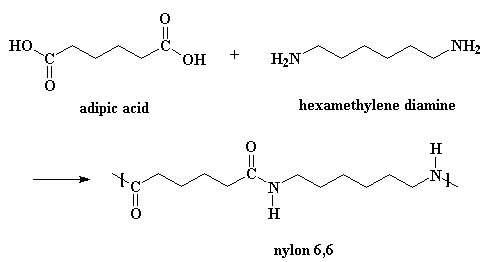
Of course, there are a few more details to the reaction than you see up there in that little picture.
To make nylon 6,6 one doesn't need a catalyst, but acids do catalyze the reaction, and wouldn't you know it, one of the monomers is itself an acid. A little reaction happens between two adipic acid molecules. One will donate a proton to a the carbonyl oxygen of another to get things started.
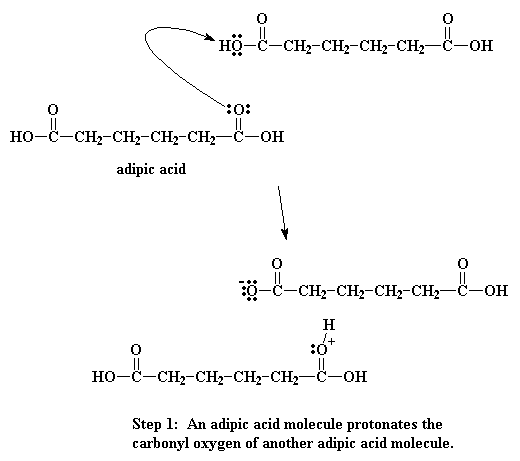
When this oxygen is protonated the carbonyl oxygen becomes much more vulnerable to attack by the nitrogen of our diamine. This happens because our protonated oxygen bears a positive charge. Oxygen does not like to have a positive charge. So it pulls the electrons it shares with the carbonyl toward itself. This leaves the carbonyl carbon lacking electrons, and ready for the amine nitrogen to donate a pair to it:
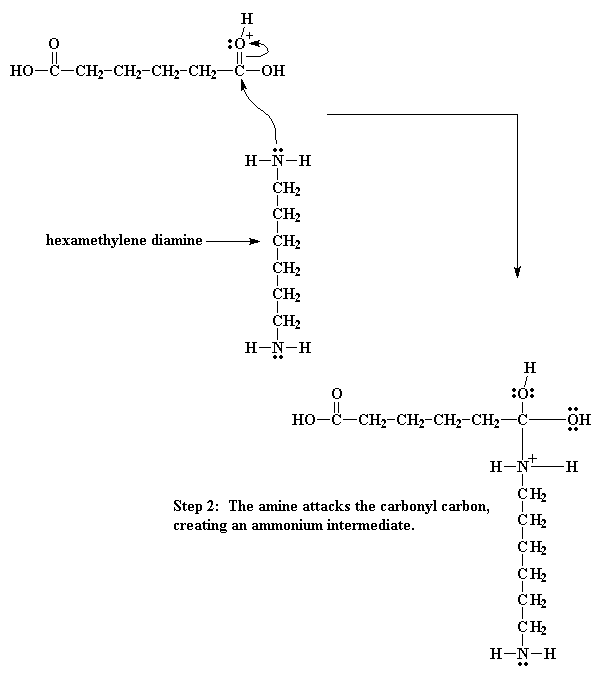
Then the electrons play musical chairs. One of the electron pairs form the carbonyl double bond shifts entirely to the oxygen, taking care of the problem of the positive charge at that atom, but now our nitrogen has a positive charge.
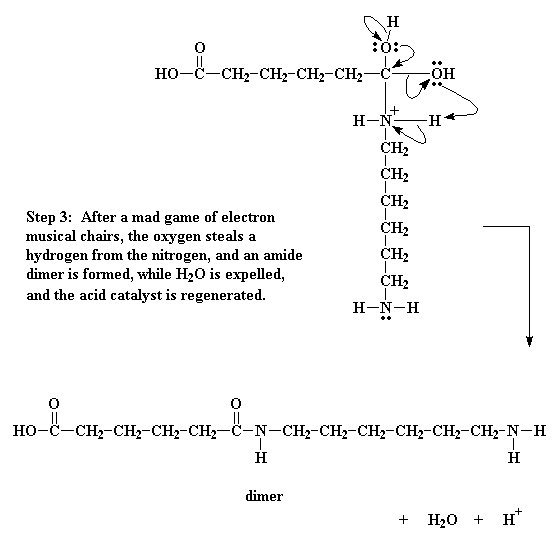
So then we get an even more elaborate game of electron musical chairs. The electrons from the hydrogen oxygen bond go back to the oxygen, freeing the proton, regenerating the acid catalyst. Then the carbonyl oxygen shares its newly regained electrons with the carbon atoms, regenerating the carbonyl double bond.
Of course, this isn't enough. The oxygen of the hydroxyl group decides to do a little bit of electron shuffling of its own. It takes the pair it shares with the carbon and hogs them to itself, severing the bond between it and the carbon. It then donates a pair of electrons to a hydrogen connected to the nitrogen.
That gets this hydrogen thinking. As it now shares a pair of electrons with the oxygen, it sees no need to keep the pair it shares with the nitrogen, so it lets go of that pair, giving it over to the nitrogen. This shift of electrons breaks the bond between the hydrogen and the nitrogen, and gets rid of the positive charge on that nitrogen. It splits off H2O, and generates the amide-containing dimer.
So what does this dimer do? Look close, and you'll see that it has an acid group at one end, and an amine group at the other. This means that it can react with a molecule of the diacid, or a molecule of the diamine. Either way, you get a trimer.
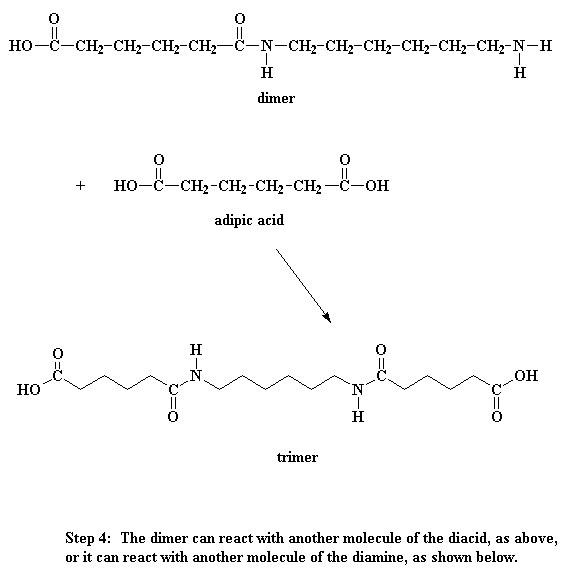
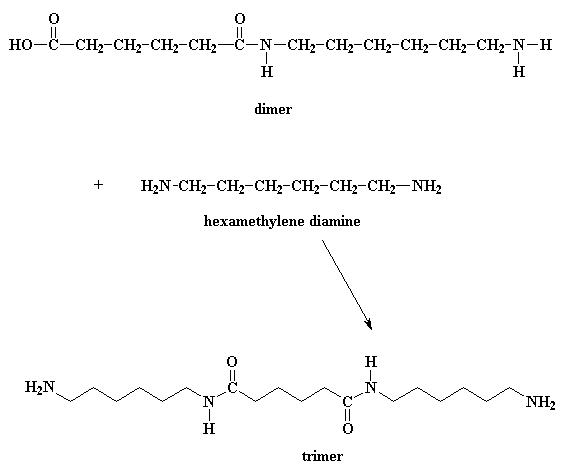
Wanna know a little secret? Our dimer can also react with other dimers, to make a tetramer if it wants to. Or it can react with a trimer to form a pentamer, and it can also react with bigger oligomers. Eventually, when this happens, dimers will grow into trimers, tetramers, and bigger oligomers, and these big oligomers will react with each other, to form even bigger oligomers. This keeps happening until they become big enough to be called polymers.
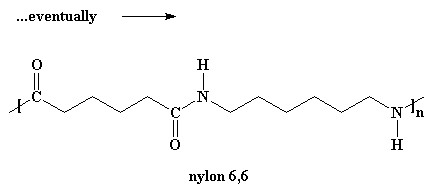
For the molecules to grow big enough to be called polymers, we have to do this reaction under a vacuum. When we do this, all that by-product water will evaporate and get sucked away. We need to get rid of the water because of a little rule called Le Chatelier's Principle.
Now remember how at the beginning of this little lesson I said that the reaction doesn't need an acid catalyst to take place? The reason we know this is that near the end of the polymerization, when there aren't many acid groups left to be catalysts, the reaction still goes on. You see, the amine can react with the unprotonated carboxylic acids. If this were not so, high molecular weight nylon 66 could not be made without an external catalyst, because the reaction would stop at higher conversions, when there aren't many acid groups left to be catalysts.
Wanna know something else?
Nylons can also be made from a diamine and a diacid chloride:
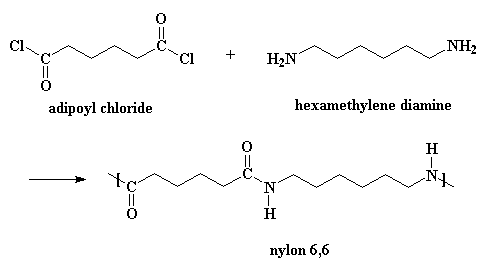
This reaction goes by the same mechanism, but you need to add a little bit of acid to act as a catalyst. (When you make nylon the other way, adipic acid acts as the catalyst.) Also, it produces HCl gas as a byproduct rather than water. If you want to see a movie of this method, click here.
There's an amazing demonstration of this reaction called (oddly enough) "The Nylon Rope Trick." And as you might have guessed from the name, the product is actually rope-like (or more accurately, string-like) that can be pulled up from the interface of the reacting solutions and wound on a rod or even a motorized take-up reel. But I'm getting ahead of myself. To find out more about this demonstration of interfacial step-growth polymerization to give a nylon, go to this page.
Tested Nylon 6,10 Syntheses
Now, if you'd actually like to make a nylon the way it's (not) made industrially, we have a procedure for you to follow. It used a diacid chloride similar to the one discussed above. It's the same one used in the nylon rope trick: click here.And for a method using sebacoyl chloride in interfacial synthesis of nylon 6,10 that gives a granular or powdery product that's much cleaner and easier to characterize, click here to view the procedure and here to download a copy.
Just to be sure you've actually made the polymer, why not get an NMR spectrum or two of your isolated product. Go here to view a 1H spectrum and and here to see the 13C spectrum.

|
Return to Nylon Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
