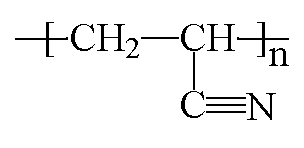
The model on the right above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the
3D model in it when you are ready to come back here.
Polyacrylonitrile is used for very few products an average consumer would be familiar with, except to make another polymer, carbon fiber. Homopolymers of polyacrylonitrile have been uses as fibers in hot gas filtration systems, outdoor awnings, sails for yachts, and even fiber reinforced concrete. But mostly copolymers containing polyacrylonitrile are used as fibers to make knitted clothing, like socks and sweaters, as well as outdoor products like tents and such. If the label of some piece of clothing says "acrylic", then it's made out of some copolymer of polyacrylonitrile. Usually they're copolymers of acrylonitrile and methyl acrylate, or acrylonitrile and methyl methacrylate:
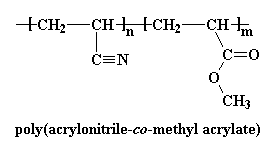
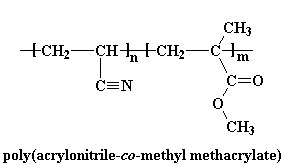
Also, sometimes we make copolymers of acrylonitrile and vinyl chloride. These copolymers are flame-retardant, and the fibers made from them are called modacrylic fibers.
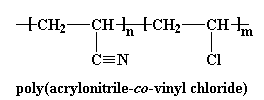
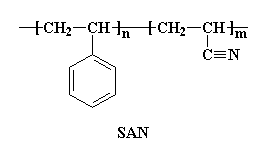
But the slew of copolymers of acrylonitrile doesn't stop there. Poly(styrene-co-acrylonitrile) (SAN) and poly(acrylonitrile-co-butadiene-co--styrene) (ABS), are used as plastics.
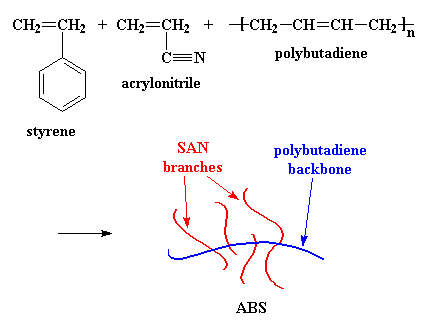
 ABS is very strong and lightweight. It is strong enough to be used to
make automobile body parts, but it is so light that Wassana can lift this
front bumper fascia over her head with only hand! Using plastics like ABS
makes automobiles lighter, so they use less fuel, and therefore they
pollute less.
ABS is very strong and lightweight. It is strong enough to be used to
make automobile body parts, but it is so light that Wassana can lift this
front bumper fascia over her head with only hand! Using plastics like ABS
makes automobiles lighter, so they use less fuel, and therefore they
pollute less.
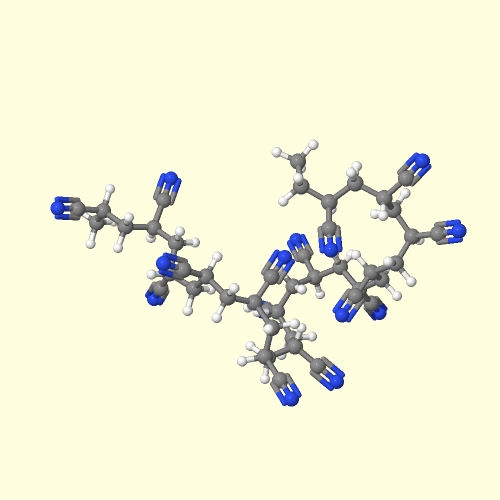
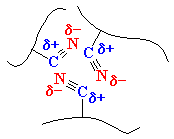 ABS is a stronger plastic than polystyrene
because of the nitrile groups of its acrylonitrile units. The
nitrile groups are very polar, so they are attracted to each other. This
allows opposite charges on the nitrile groups to stabilize each other
like you see in the picture on the left. This strong attraction holds ABS
chains together tightly, making the material stronger. Also the rubbery
polybutadiene makes ABS tougher than polystyrene.
ABS is a stronger plastic than polystyrene
because of the nitrile groups of its acrylonitrile units. The
nitrile groups are very polar, so they are attracted to each other. This
allows opposite charges on the nitrile groups to stabilize each other
like you see in the picture on the left. This strong attraction holds ABS
chains together tightly, making the material stronger. Also the rubbery
polybutadiene makes ABS tougher than polystyrene.
A derivative of acrylonitrile has TWO nitrile groups on the same vinyl carbon. It's
called cyanoacrylonitrile or dicyanoethylene. This monomer
is extremely reactive so the polymerization is hard to control.
The polymer made from this monomer isn't used for anything that we
know of, but then, maybe someday?
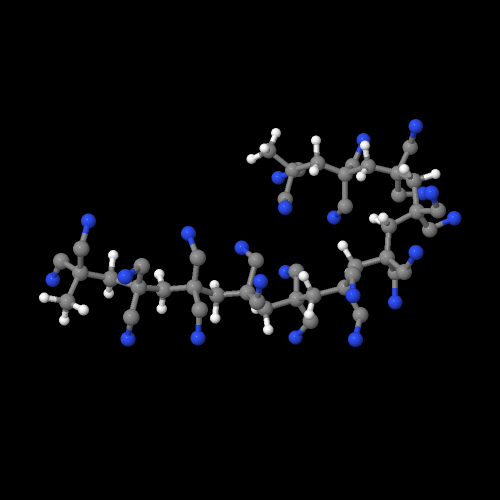
The model above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the
3D model in it when you are ready to come back here.
Polyacrylonitrile is a vinyl polymer, and a derivative of the acrylate family of polymers. It is made from the monomer acrylonitrile by free radical vinyl polymerization.

This is what the monomer acrylonitrile really looks like:
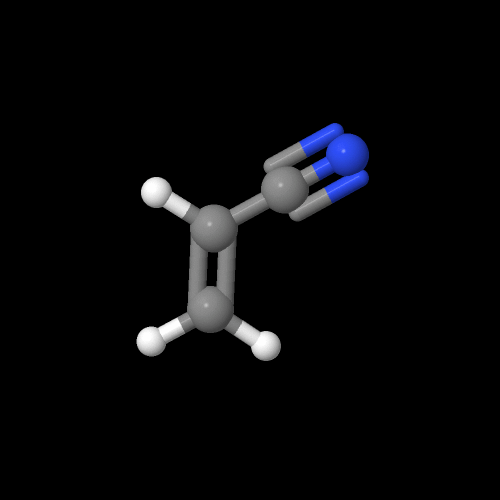
The model above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the
3D model in it when you are ready to come back here. the new window that opens up with the 3D model in it when you are ready to come back here.
Tested Polyacrylonitrile Syntheses
Despite the fact that acrylonitrile is pretty dangerous healthwise, you may still want to make your own sample for some reason. We actually have TWO procedures you can try. One goes free radically, the other anionically. Volatile acrylonitrile requires extreme care in the lab to be safe: be careful!The first method is the slurry radical one: click here to see the procedure and here to download a copy.
The second method uses calcium oxide initiator: click here to see the procedure and here to download a copy.
Other polymers that are used as fibers include:

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
