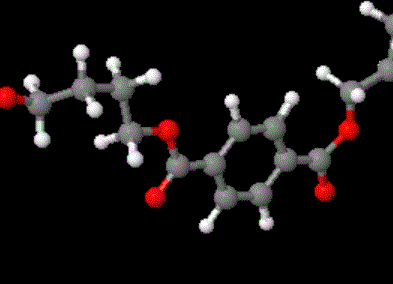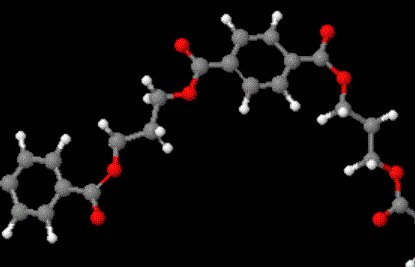
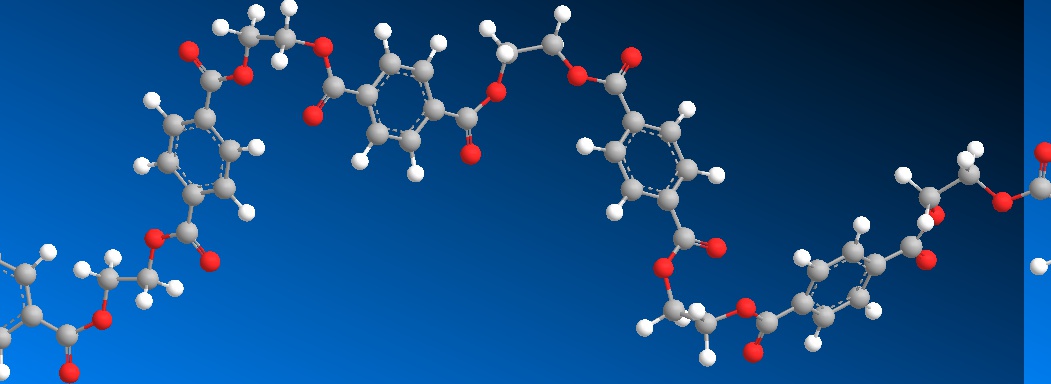
The model above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
 Polyesters are the polymers, in the form of fibers, that were used back in the seventies
to make all that wonderful disco clothing, the kind you see being modeled
on the right. But since then, the nations of
the world have striven to develop more tasteful uses for polyesters, like
those
nifty shatterproof plastic bottles that hold your favorite refreshing
beverages, like the blue bottle in the picture below. So you
see, polyesters can be both plastics and fibers. Another place you find polyester is in
balloons. Not the cheap ones that you use for water balloons, those are
made of natural rubber. I'm talking about
the fancy ones you get when you're in the hospital. These are made of
a polyester film made by DuPont called Mylar. The balloons are made of a
sandwich, composed of Mylar and aluminum foil.
Materials like this, made
of two kinds of material, are called composites.
Polyesters are the polymers, in the form of fibers, that were used back in the seventies
to make all that wonderful disco clothing, the kind you see being modeled
on the right. But since then, the nations of
the world have striven to develop more tasteful uses for polyesters, like
those
nifty shatterproof plastic bottles that hold your favorite refreshing
beverages, like the blue bottle in the picture below. So you
see, polyesters can be both plastics and fibers. Another place you find polyester is in
balloons. Not the cheap ones that you use for water balloons, those are
made of natural rubber. I'm talking about
the fancy ones you get when you're in the hospital. These are made of
a polyester film made by DuPont called Mylar. The balloons are made of a
sandwich, composed of Mylar and aluminum foil.
Materials like this, made
of two kinds of material, are called composites.
 A special family of polyesters are polycarbonates.
A special family of polyesters are polycarbonates.
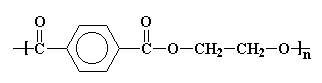
Polyesters have
hydrocarbon backbones which contain ester linkages, hence the name.
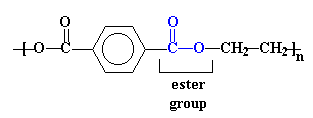
The structure in the picture is called poly(ethylene terephthalate), or
PET for short, because it is made
up of ethylene groups and terephthalate groups (duh!). I realize that
terephthalate is not the kind of word most English-speaking mouths
are used to saying, but with practice you should be able to say it with
only a slight feeling of awkwardness when it rolls off your tongue.
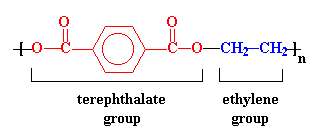
The ester groups in the polyester chain are polar, with the carbonyl oxygen atom having a somewhat negative charge and the carbonyl carbon atom having a somewhat positive charge. The positive and negative charges of different ester groups are attracted to each other. This allows the ester groups of nearby chains to line up with each other in crystal form, which is why they can form strong fibers.
The inventor who first discovered how to make bottles from PET was Nathaniel Wyeth. He's the brother of Andrew Wyeth the famous painter. But others had tried before. Go read this story of someone who may have been the first person to try to make a shatterproof bottle.
Now I'm sure everyone out there is just dying to have two questions
answered. The first one is:
Why can't you return plastic soft drink bottles to get a cool nickel per bottle like you could with the old glass bottles?
And the second one which I'm positive everyone is wondering about is:
How come peanut butter comes in neato shatterproof jars but jelly doesn't?
These two riveting questions, as it turns out, have the same answer. The answer is that PET has too low a glass transition temperature, that is the temperature at which the PET becomes soft. Now reusing a soft drink bottle requires that the bottle be sterilized before it is used again. This means washing it at really high temperatures, temperatures too high for PET. Filling a jar with jelly is also carried out at high temperatures. Down at your local jelly factory, the stuff is shot into the jars hot, at temperatures which would cause PET to become soft. So PET is no good for jelly jars.
PEN Saves the Day!
There is a new kind of polyester that is just the thing needed for jelly jars and returnable bottles. It is poly(ethylene naphthalate), or PEN. Click on the model on the right for a 3D version.
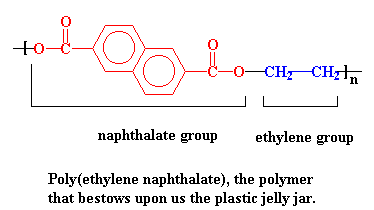
PEN has a higher glass transition temperature than PET. That's the temperature at which a polymer gets soft. The glass transition temperature of PEN is high enough so that it can withstand the heat of both sterilizing bottle washing and hot strawberry jelly. PEN is so good at standing the heat that you don't even have to make the bottle entirely out of it. Just mixing some PEN in with the old PET gives a bottle that can take the heat a lot better than plain old PET.
All right, this polyester stuff is the best thing since
sliced bread.
Agreed. But how does one make it?
Ok here goes...
In the big plants where they make polyester, it's normal to start off
with a compound called dimethyl terephthalate. This is reacted with
ethylene glycol is a reaction called transesterification. The
result is bis-(2-hydroxyethyl)terephthalate and methanol. But if we heat
the reaction to around 210 oC the methanol will boil away and
we don't
have to worry about it anymore.
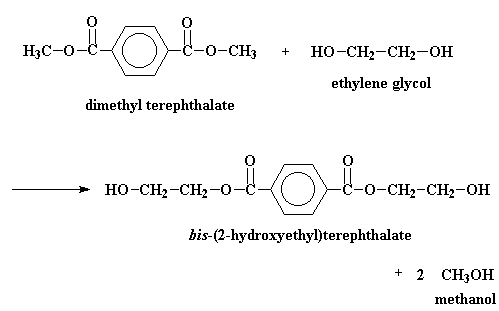
Then the bis-(2-hydroxyethyl)terephthalate is heated up to a balmy 270 oC, and it reacts to give the poly(ethylene terephthalate) and, oddly, ethylene glycol as a by product. Funny, we started off with ethylene glycol.

If you want to know how all these reactions go down, click here.
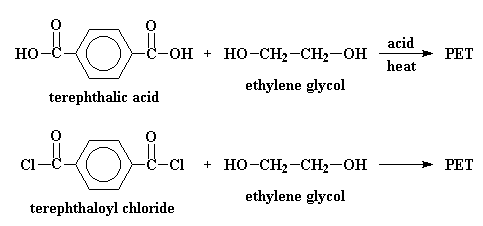
But in the laboratory, PET is made by other reactions. Terephthalic acid
and ethylene glycol can polymerize to make PET when you heat them with an
acid catalyst. It's possible to make PET from terephthoyl chloride and
ethylene glycol. This reaction is easier, but terephthoyl chloride is
more expensive than terephthalic acid, and it's a lot more dangerous.
There are two more polyesters on the market that are related to PET. There is poly(butylene terephthalate) (PBT) and poly(trimethylene terephthalate). They are usually used for the same type of things as PET, but in some cases these perform better.
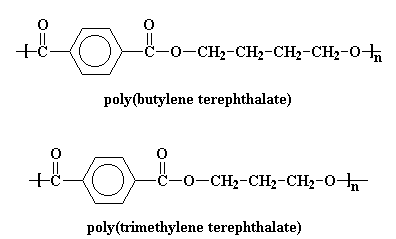
Click on either of the two images above to pop up a 3D version of PBT (on the left) ad PTT (right). You can rotate, expand and convert to stick-and-ball structures, for example. Fun!
NMR Spectra of PET and PBT
You somehow find what you think is a sample of one of these wonderful polyesters. How can you be sure that's what it is? Why not get an NMR spectrum or two? But of course, you have to have an actual spectrum of this material to compare it to.So here's a 1H spectrum of PET and and here's its 13C spectrum.
And if you think you might actually have PBT instead, here's a 1H spectrum of PBT and and here's its 13C spectrum.
Other polymers used as plastics:| |
Other polymers used as fibers: | |
| Polypropylene | Polypropylene | |
| Polyethylene | Polyethylene | |
| Polystyrene | Nylon | |
| Polycarbonate | Kevlar and Nomex | |
| PVC | Polyacrylonitrile | |
| Nylon | Cellulose | |
| Poly(methyl methacrylate) | Polyurethanes |

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |