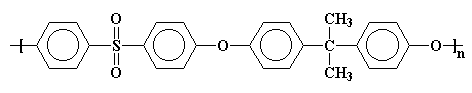
The model above is an image of the pdb model
you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
Poly(ether sulfones) also can stand up well to water and steam, so they're used to make things like cookware and medical products that need to be sterilized between uses.
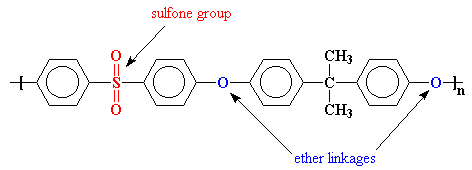
Poly(ether sulfones get their name because they have ether groups and sulfone groups in their backbone chains. PES polymers have high glass transition temperatures, or Tg's, because the sulfone groups are so stiff. In fact, poly(phenyl sulfone) is so stiff that it doesn't have a glass transition temperature! It stays hard as a rock right up until it decomposes at around 500 oC.
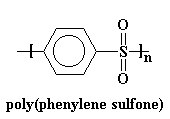
This is bad. This means that it can't be processed. In order to make it processable, we have to make that chain a little more flexible so the polymer will soften at a reasonable temperature. We do this by putting flexible groups in the backbone chain, namely ether linkages.
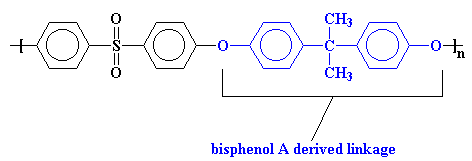
UdelTM uses a bisphenol A derived linkage to make the chain more flexible. You'll notice that this polymer has two ether linkages in the repeat unit. For this reason UdelTM is more properly called a poly(ether ether sulfone).
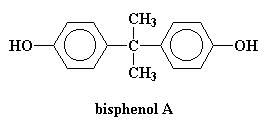
We call this a bisphenol A linkage because it's based on the monomer bisphenol A, but more about that later.
This brings the Tg down to 190oC. By playing around and using different kinds of flexible groups we can alter the Tg further. If we use a less flexible linkage, the Tg jumps to 230 oC.
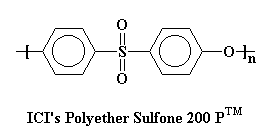
This PES is made by Imperial Chemical Industries (ICI), and they call it by the romantic name of Polyether Sulfone 200 PTM. You'll notice that, unlike UdelTM, this polymer has only one ether linkage between sulfone groups. This makes the chain stiffer and the Tg higher.
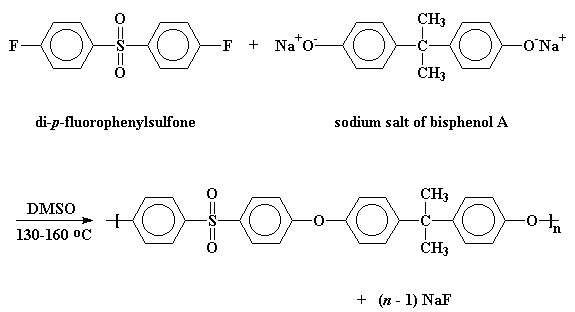
But you ask, "How do we make such a wonderful poly(ether sulfone)?" Like this: One first forms the sodium salt of bisphenol A (remember bisphenol A from polycarbonate?) and then reacts it with di-para-fluorophenylsulfone in a solvent like dimethylsulfoxide (DMSO) at between 130 and 160 oC and that gets us a poly(ether sulfone)!
It's actually much harder than it sounds. You have to keep any water out of the solvent and away from the reaction, or a trace of hydoxide ion can react instead of the phenol anion. This kills the reaction and limits the molecular weight. You also have to keep oxygen out of the reaction because this also can cause undesirable oxidation to occur. In any event, industrial scientists figured this all out because we actually DO have these polymers made on an industrial scale.
NMR Spectra of Udel Polysulfone
You somehow get your hands on what you think is a sample of this wonder polymer. How can you be sure that's what it is? Why not get an NMR spectrum or two? But of course, you have to have an actual spectrum of this material to compare it to: go here to view a 1H spectrum and and here to see the 13C spectrum.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
