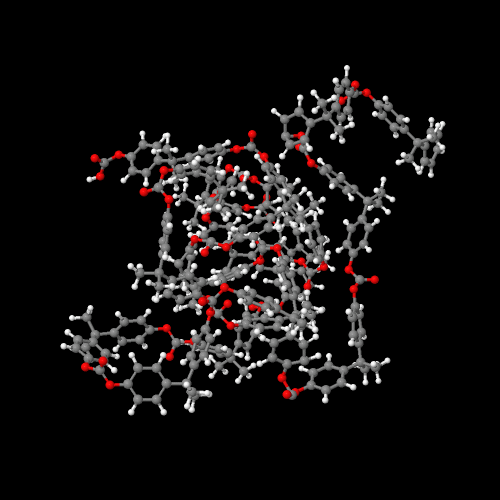
Click on this link to the 3D model or just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
Polycarbonate, or specifically polycarbonate of bisphenol A, is a clear plastic used to make shatterproof windows, lightweight eyeglass lenses, and such. General Electric makes this stuff and sells it as Lexan.
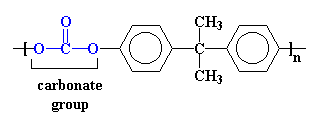
Polycarbonate gets its name from the carbonate groups in its backbone chain. We call it polycarbonate of bisphenol A because it is made from bisphenol A and phosgene. You can see them below in the regular way we draw molecules or see them in 3-D further below.
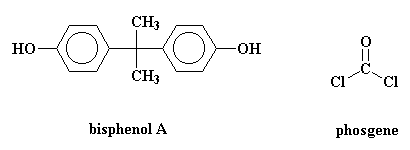
The model on the left below is an image of the BPA pdb model you can view by clicking here or you can just click on the image itself. The second image is of the diacid chloride monomer phosgene. Either way, be sure to close the new windows that open up with the 3D model in it when you are ready to come back here.
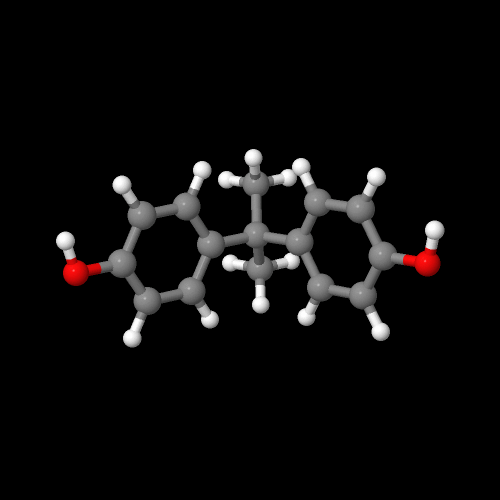
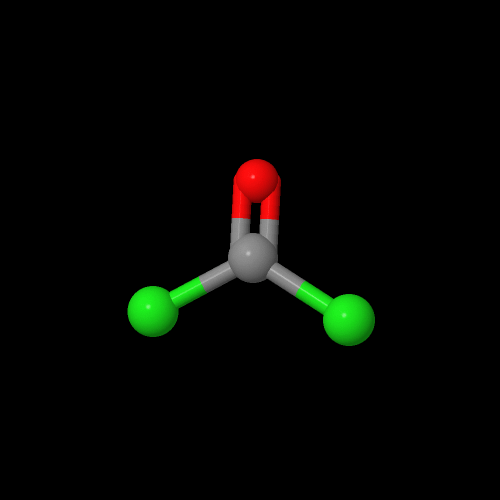
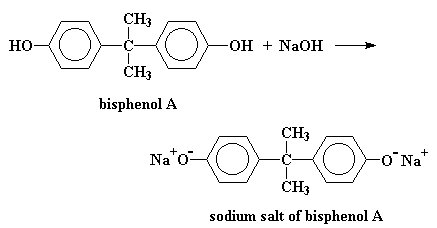
The first step in the synthesis of polycarbonate starts out with the reaction of bisphenol
A with sodium hydroxide to get the sodium salt of bisphenol A.
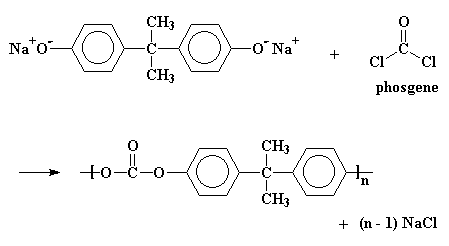
The sodium salt of bisphenol A is then reacted with phosgene, a right
nasty compound which was a favorite chemical weapon in World War I, to
produce the polycarbonate.
What? You want the gritty details of the reaction? Then click here and you will not be disappointed. We give you all the steps of the mechanism in gruesome detail.
Another polymer used for unbreakable windows is poly(methyl methacrylate).
Seeing Another Polycarbonate More Clearly
Up until now, we've been talking about only one polycarbonate, polycarbonate of bisphenol A. But there's another polycarbonate out there, that some of us look at all the time. In fact, some of us, like me, never look at anything without the help of this polycarbonate. This is the polycarbonate that is used to make ultra-light eyeglass lenses. For people with really bad eyesight, like me, if the lenses were made out of glass, they would be so thick that they'd be too heavy to wear. I know. I used to have glass lenses. My glasses were so heavy that wearing them gave me a headache. But this new polycarbonate changed all that. Not only is it a lot lighter than glass, but it has a much higher refractive index. That means it bends light more than glass, so my glasses don't need to be nearly so thick.So what is this wonderful new polycarbonate? It's very different from polycarbonate of bisphenol A. We make it by starting with this monomer:
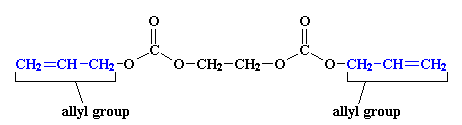
You can see that it has two allyl groups on the ends. These allyl groups have carbon-carbon double bonds in them. This means they can polymerize by free radical vinyl polymerization. Of course, there are two allyl groups on each monomer. The two allyl groups will become parts of different polymer chains. In this way, all the chains will become tied together to form a crosslinked material that looks like this:
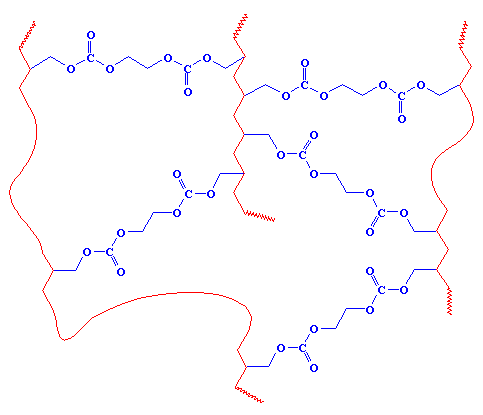
As you can see, the carbonate-containing groups (shown in blue) for the crosslinks between the polymer chains (shown in red). This crosslinking is makes the material very strong, so it won't break nearly as easily as glass will. This is really important for kids' glasses! If only this stuff had been invented when I was a kid!
There is a fundamental difference in the two types of polycarbonate described here that I should point out. Polycarbonate of bisphenol A is a thermoplastic. This means it can be molded when it is hot. But the polycarbonate used in eyeglasses is a thermoset. Thermosets do not melt, and they can't be remolded. They are used to make things that need to be really strong and heat resistant.
Other polymers used as plastics include:
Other polymers used as thermosets
include:
For more information, at other websites...
-
Baffled by the Baby Bottle: A Case Study in Chemistry - an activity which
can be used as a compliment or an alternative to The Great PVC Controversy, dealing with
the safety of polycarbonate baby bottles, created by Michael A. Jeannot Department of
Chemistry, St. Cloud State University, hosted by the State University of New York at Buffalo.

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
