Le Chatelier's principle
oligomer
transesterification
I'm taking a wild guess that because you're reading this page, you a) don't know how polyesters are made and b) you want to know. So here goes:
To make polyester, one starts off with two compounds. If you've read the polyester page, you know that they're ethylene glycol and dimethyl terephthalate, although the real intermediate in the polymerization is the bis(2-hydroxyethyl) terephthalate. If you want to see these two in 3-D, click here.
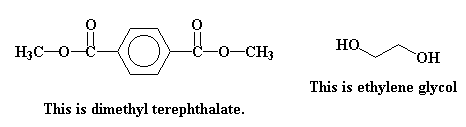
Something fun happens when these two molecules come together. You see, dimethyl terephthalate is an ester, and ethylene glycol is a alcohol. When alcohols and esters come together they perform little dance of a reaction called transesterification.
This is one of those dances where a lot of changing partners goes on. Take a look at one of the methoxy groups (in red) on dimethyl terephthalate, and the hydroxyethoxy group (in blue) of the ethylene glycol. These two groups are going to trade places, you see. The hydroxyethoxy group is going to end up on the ester, where the methoxy group had been. The methoxy group is going to end up attached to a hydrogen, where the hydroxyethoxy group had been.
Isn't hydroxyethoxy a fun word to say? Go ahead, try it!
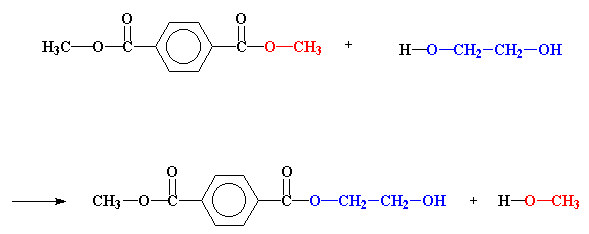
That's what happens in the first step of making a polyester. Let's take a look at just how this happens.
The important place to look right now is at the ester, the dimethyl terephthalate. Specifically, look at the carbonyl carbon. Notice that it's double bonded to an oxygen. Oxygen is a lot more electronegative than carbon, so it will pull electrons away from the carbon. This leaves the carbon with a partial positive charge. This means that it can be very easily attacked by a pair of electrons that comes along.
Now just where would a pair of electrons come from?
When there's an alcohol around, like ethylene glycol, the unshared pairs of electrons on the alcohol oxygen atoms will do nicely. These attack just as you see in the picture below.
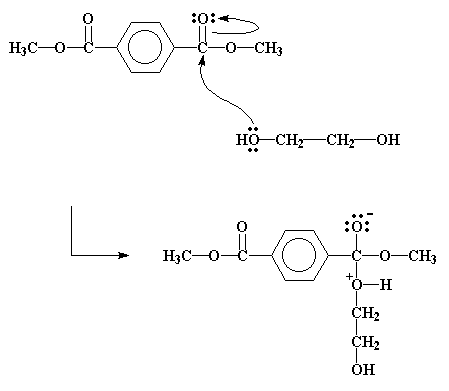
And so it happens that the methyl group and the hydroxyethyl group end up trading places.
That methanol is a byproduct. We don't want it around. If we run this reaction at a high enough temperature, the methanol will boil away. This is good. It not only gets rid of the methanol, but removing this product of the reaction drives the transesterification reaction to higher conversions. Remember Le Chatelier's principle?
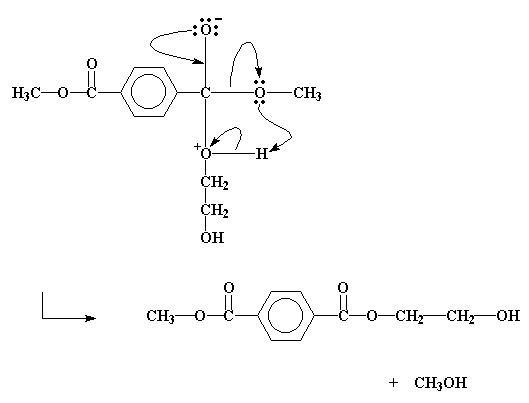
transesterification reaction, click here.
The same reaction will happen on the other side of the terephthalate, and this give us bis-(2-hydroxyethyl)terephthalate.
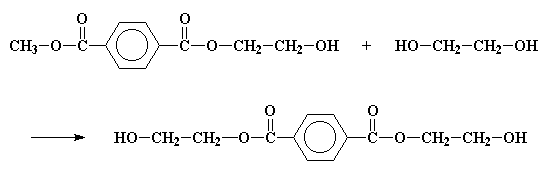
If you want to see bis-(2-hydroxyethyl)terephthalate in 3-D, here.
After we've made the bis-(2-hydroxyethyl)terephthalate, it does some more reacting. But these reactions are easy to follow, because they're all transesterifications similar to the one you just saw. Take a look. This molecule is an alcohol and an ester! So it can transesterify with itself, as the picture beneath shows.
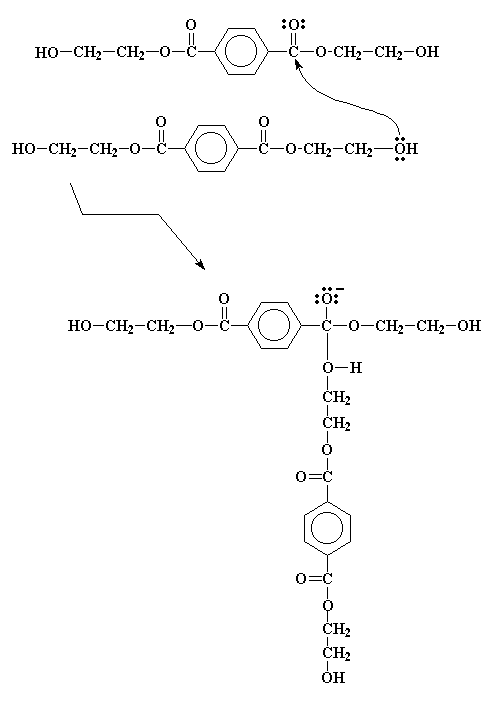
It forms an intermediate, similar to the one we saw earlier, and no surprise, it goes through a similar electron shuffle to get the product we see in the picture right below.
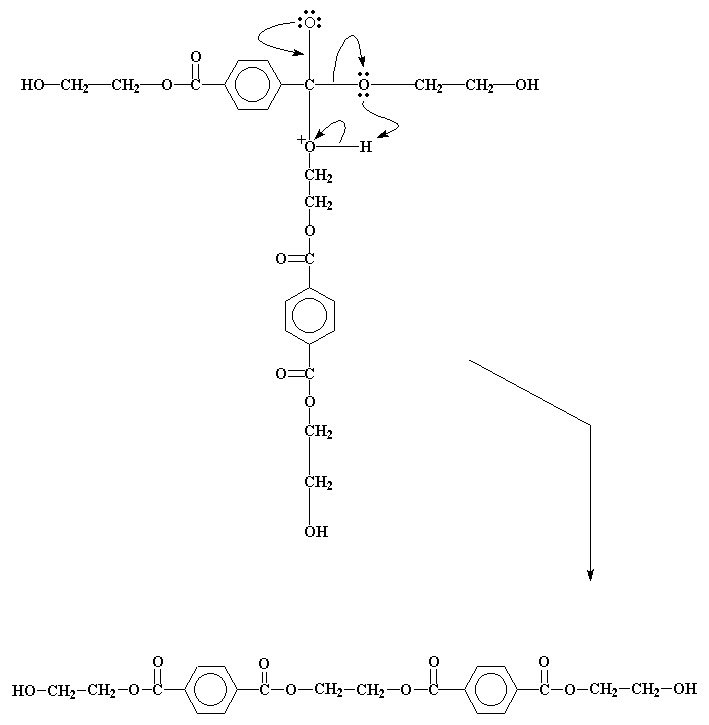
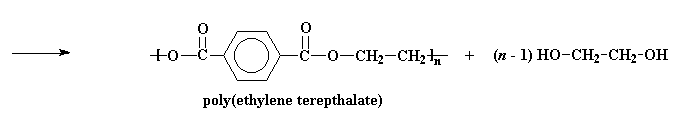
This product can react again, just like the bis-(2-hydroxyethyl)terephthalate did. As this happens, the molecules formed get bigger and bigger, until we get high molecular weight PET!
Tested Synthesis of PET Polyester
So if you'd actually like to make PET the way it's made in the lab, we have a procedure for you to follow. It uses the dimethyl ester of terephthalic acid and ethylene glycol, very similar to what is used industrially. This is a safe and reproducible synthesis- just be careful like always when working in the lab.Click here to see the procedure and here to download a copy.
NMR Spectra of PET and PBT
You somehow find what you think is a sample of one of these wonderful polyesters. Maybe you even made it yourself. How can you be sure that's what it is? Why not get an NMR spectrum or two? But of course, you have to have an actual spectrum of this material to compare it to.So here's a 1H spectrum of PET and and here's its 13C spectrum.
And if you think you might actually have PBT instead, here's a 1H spectrum of PBT and and here's its 13C spectrum.

|
Return to the Polyester Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |
