
The Tau of Tacticity
I suppose that if we're going to have a page about something called "tacticity", it might be a good idea to let you know just what tacticity is. Tacticity is simply the way pendant groups are arranged along the backbone chain of a polymer. We talk about tacticity a lot when dealing with vinyl polymers. To illustrate tacticity, we're going to start with one of those vinyl polymers, our good friend polystyrene, which you might now know is usually "amorphous."
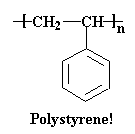
Now, polystyrene is often drawn as a flat picture like this:
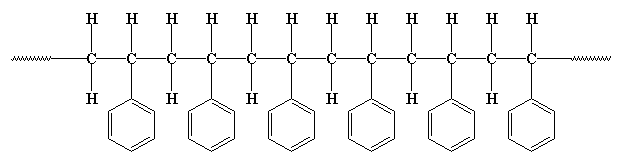
But polymers aren't really flat like that. The carbon atoms aren't really in such a straight, nor are the hydrogens and phenyl groups all placed at perfect right angles. The carbon chain is more of a zigzag like this:
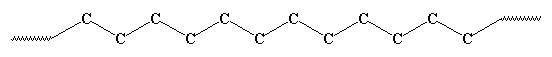
The pendant groups tend to be point away from the chain, like this:
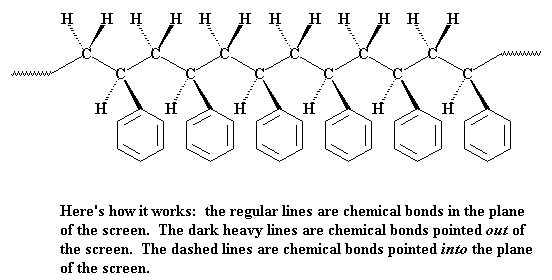
In that picture you see all the phenyl groups are located on the same side of the polymer chain. But they don't have to be this way. To illustrate let's look at a chain of polystyrene from above in the figure below. You can see that the pendant phenyl groups can be either on the right or left side of the chain. If all of the phenyl groups are on the same side of the chain, we say the polymer is isotactic. If the phenyl groups come on alternating sides of the chain, the polymer is said to be syndiotactic. If the phenyl groups are on both sides, right and left, but in no particular order, in a random fashion, than we say the polymer is atactic.
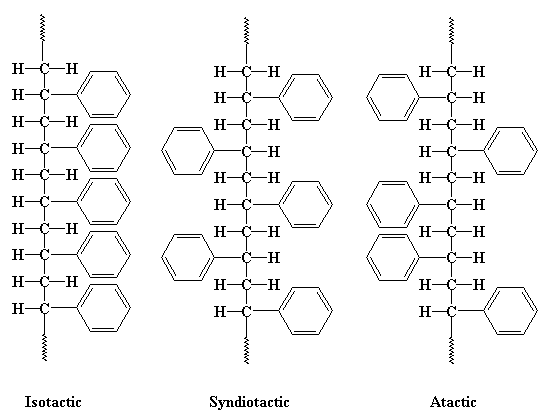
If you want to get a look at this in 3-D, click here. Note: Isotactic polystyrene isn't produced commercially. We're just using it here as a hypothetical example. Other polymers can be isotactic, though, and we'll talk about those later.
What does this have to do with anything, you ask? A lot! You see, when polymers have a regular arrangement of their atoms, like we see in the isotactic and syndiotactic polystyrenes, it is very easy for them to pack together into crystals and fibers. But if there is no order, as is the case with the atactic polystyrene, packing can't occur easily or at all. This is because molecules pack best with other molecules of the same shape and size. Try packing a box full of identical objects, and then one with very different objects, and you'll get the idea.
Ziegler and Natta Come to the Rescue
This can be a big problem for some polymers. Tacticity makes a big difference in polystyrene, for one. And, as you'll find out, how we make a particular polymer determines it's tacticity. Free radical vinyl polymerization normally can only produce atactic polymers. But this is by no means bad. Atactic polystyrene is a hard plastic, and completely amorphous, but it's still very useful. It can't crystallize at all, but it has a glass transition temperature of over 100 oC which means it can be used in lots of applications. More recently, we discovered metallocene catalysis vinyl polymerization. Using this polymerization method, syndiotactic polystyrene became possible. It's not only crystalline, but it doesn't melt until 270 oC, way higher than the thermal softening temperature of the atactic version.Another vinyl polymer, polypropylene, is a good example of the effects of tacticity. At first, there was only atactic polypropylene made by free radical polymerization. It's kind of soft and sticky, not very strong, and not much good for anything. Then along came two scientists named Ziegler and Natta, one German and the other Italian. They invented a new type of vinyl polymerization which ended up being named Ziegler-Natta polymerization. This new process could make isotactic polypropylene, which was an incredible discovery. This new polypropylene could crystallize (a lot), and could be used to make fibers for things like indoor-outdoor carpeting.
It also allowed an important revolution in kitchen storage containers. For a long time, clear plastic food containers were made of polyethylene. They worked great, keeping food (especially left-overs) safe in the frig. Then along came dishwashers with their high temperature drying and sterilization cycles. Bingo! A new form of plastic art was born! Polyethylene containers twisted and turned, and mostly shrank, till they formed delightful shapes that were absolutely useless as containers.
Polypropylene, the new isotactic kind, had a much higher melting point than polyethylene. Bingo, again! Food containers made with this new polymer were "dishwasher safe." And we all know, that means a lot to the average homemaker. In fact, polypropylene is used for all kinds of containers, tubes, gas lines, water lines in refrigerators and dishwashers, and tons of other things. Thank you, Ziegler and Natta!

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |