
Making Epoxy Monomers and Polymers
There are two steps to making epoxy resins. First you have to make a diepoxy, and then you have to crosslink it with a diamine. So we're going to talk about those two steps separately. You can read about whichever you like first:
Making the Diepoxy
This step is a type of step-growth polymerization in its own right. We make the prepolymer using bisphenol A and epichlorohydrin. You can look at these molecules in 3-D by clicking here. The reaction is this, which you saw on the epoxy page:
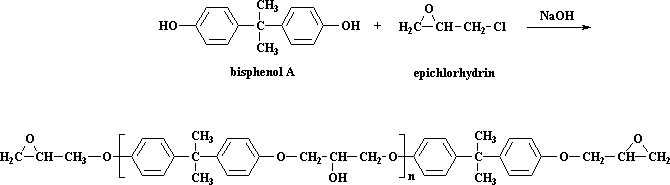
So how does this reaction work? The first thing that happens is that the NaOH does a little swap with the bisphenol A, to give the bisphenol A sodium salt:
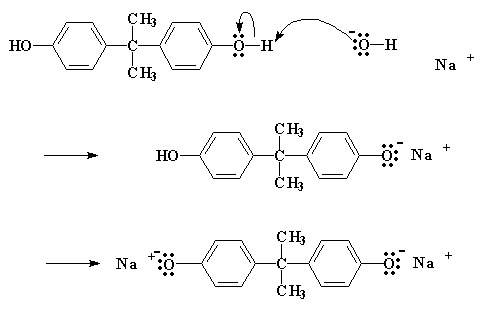
Click here to see a movie of this step of the reaction.
The salt, as you will notice, has an oxygen with three pairs of electrons that it isn't sharing with anyone. Now this particular oxygen atom is generous, and wants to share its electrons with less fortunate atoms. So it finds a carbon atom on a nearby epichlorohydrin which could use some electrons (see below). That atom is the carbon atom right next to the chlorine. The chlorine is supposed to be sharing a pair of electrons with that carbon, but being electronegative as it is, tends to hog that pair.
So the oxygen, being the kind atom it is, donates a pair of its electrons to the carbon. Carbon of course can only share four pairs of electrons at once, so one pair has to go if it wants to take the oxygen's pair. So it lets go of the electrons it has been sharing with chlorine (which really wants that pair for its own anyway), and sends the chlorine atom on its way, expelling it from the molecule.
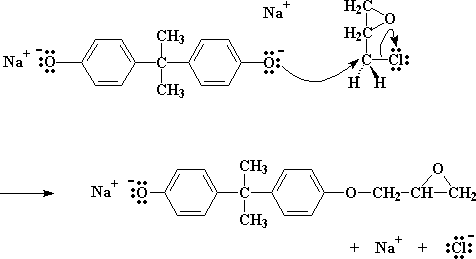
Click here to see a movie of this step of the reaction.
We end up with a molecule similar to bisphenol A, only with an epoxy group on it. And we also get NaCl.
So what happens next? Different things can happen. Remember on the epoxy page we talked about how these prepolymers come in different molecular weights. Sometimes the degree of polymerization is as high as 25. Sometimes they can be as small as this:

How big or small the prepolymer gets depends on the ratio of epichlorohydrin to bisphenol A in the reaction mixture. Let's put that ratio at two molecules of epichlorohydrin for every molecule of bisphenol A. Let's see what happens to the molecule we just made at this ratio:
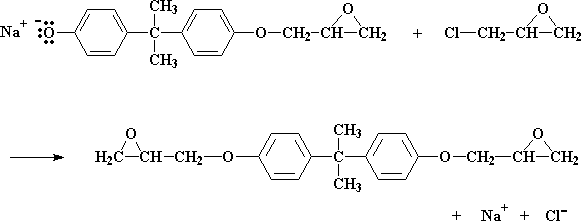
What happens is we get an epoxy group on the other side, too. The reaction then stops, as there are no more bisphenol A salt groups left to react.
But what if there are less than two molecules of epichlorohydrin for every Bisphenol A molecule? Then not all of the bisphenol A salt groups can react with epichlorohydrin. Let's say our ratio now is three molecules of epichlorohydrin for every two molecules of bisphenol A. When all the epichlorohydrin molecules have reacted we're left with a 50:50 mixture of these two molecules:
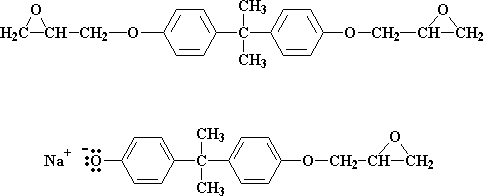
These two molecules then can react together to get this molecule:
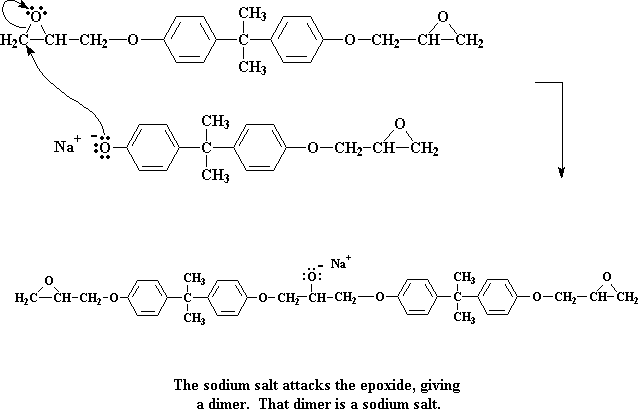
Click here to see a movie of this step of the reaction.
We're left now with a dimer, which happens to be a sodium salt. Take special note of the negative charge on the oxygen atom. When a water molecule comes along (remember, we made a bunch of water molecules when we made the sodium salt of bisphenol A) a pair of electrons from the oxygen will attack one of the water's hydrogens, stealing it from the water.
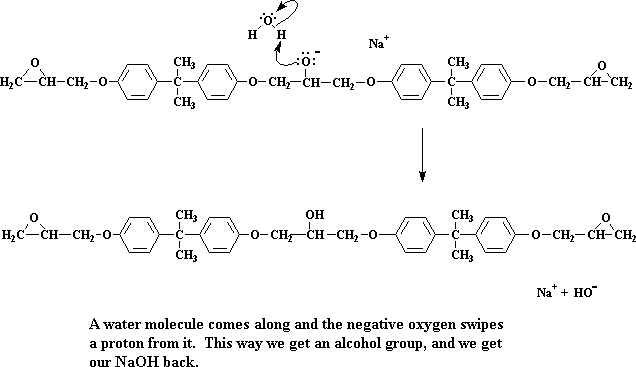
Click here to see a movie of this step of the reaction.
The oxygen now forms an alcohol group, and we get our NaOH back. Remember NaOH?
The more epichlorohydrin you have relative to the amount of bisphenol A salt, the bigger the oligomer you get. Can you figure out why? What ratio would you have to use to get trimers?
Now folk, if you want to see a movie of the whole process, click here.
Curing the Diepoxy with a Diamine
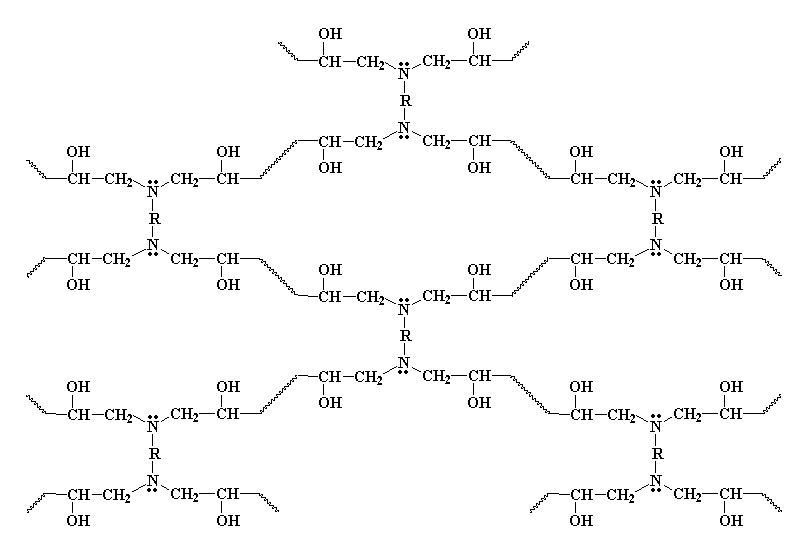
Once you've made your diepoxy prepolymers, you have to tie them all together. We do this by adding a diamine. The diamine does something funny when it sees those epoxy groups at the ends of the prepolymer. The lone pairs of electrons on the amine groups are going to look at those epoxy groups, and they're going to see that the epoxy oxygen, electronegative as it is, is sucking all the electrons away from the carbon atoms next to it. So the electrons look at those carbon atoms, and sees that they can easily give their electrons to the carbon atom on the end of the molecule.
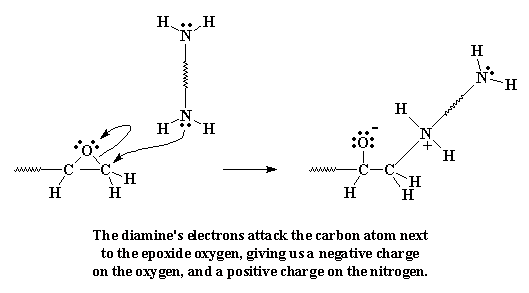
Click here to see a movie of this reaction step.
When they do this, the carbon gives up the electrons it was sharing unequally with the oxygen. The bond between the carbon and the oxygen is broken, and a new bond forms between the carbon and the amine nitrogen. We're left with a negative charge on the oxygen, and a positive charge on the nitrogen.
Everybody got that?
Take a look at that oxygen. Oxygen likes electrons, but it has three pairs that it's not sharing with any other atom. Even oxygen knows you can have too much of a good thing. So one of those non-bonding electrons looks about for something to bond with, and lo and behold, it finds the hydrogen attached to the positive nitrogen. These electrons then attack that hydrogen.
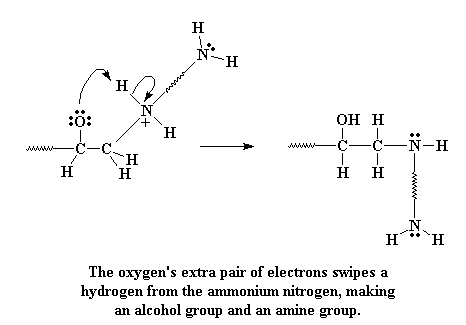
Click here to see a movie of this reaction step.
When they attack, they form a bond with the hydrogen, and the hydrogen separates from the nitrogen, leaving its electrons behind. This takes care of that positive charge, leaving the nitrogen neutral. And of course, the oxygen is now neutral too, having gained a proton, and now forms an alcohol group.
If you'd like to see a movie of the whole process, click here.
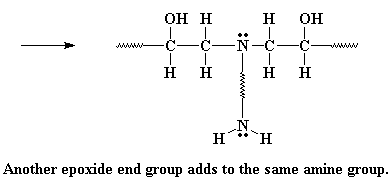
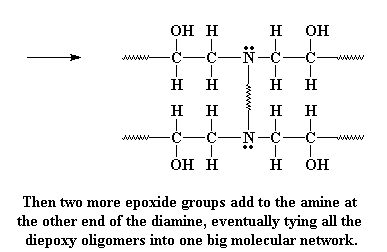
The amine group still has a hydrogen left on it, and can react with another epoxy group, in the exact same manner. As many hydrogens as the amine has, that's how many epoxy groups it can react with. Why? This is what we end up with:

|
Return to Epoxy Resins Page |

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |