The model above is an image of the polybutene model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
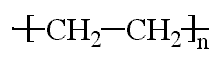
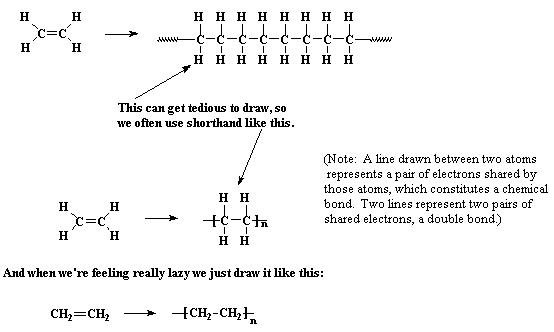
Polyethylene is probably the polymer you see most in daily life. Polyethylene is the most popular plastic in the world. This is the polymer that makes grocery bags, shampoo bottles, children's toys, and even bullet proof vests. For such a versatile material, it has a very simple structure, the simplest of all commercial polymers. A molecule of polyethylene is nothing more than a long chain of carbon atoms, with two hydrogen atoms attached to each carbon atom. That's what the picture at the top of the page shows, but it might be easier to draw it like the picture below, only with the chain of carbon atoms being many thousands of atoms long:
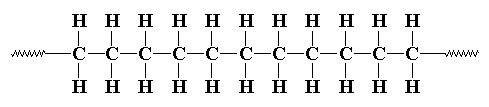
Sometimes it's a little more complicated. Sometimes some of the carbons,
instead of having hydrogens attached to them, will
have alkyl chains attached to them. These alkyl substituents are on
every other carbon as shown in the schematic below. That's because of
how the monomers (alpha-olefins as shown) are polymerized through the vinyl
group. You can't do that with typical free radical initiators like those
used to make polystyrene or poly(methyl methacrylate). No, instead you
have to use special transition metal catalysts that are usually complexed with
very special chelating groups. More on this later.
In general, these polymers are called
polyolefins. Normally, they are made from simple 1-alkenes like 1-butene,
1-hexene and so on. The odd carbon olefins are much harder to come by,
although one company in South Africa (Sasol Polymers) makes and sells them.
These include olefins like 1-pentene, 1-heptene and so on. While there are
some differences in properties between the even and odd carbon polyolefins, the
differences don't add up to earth shaking improvements.
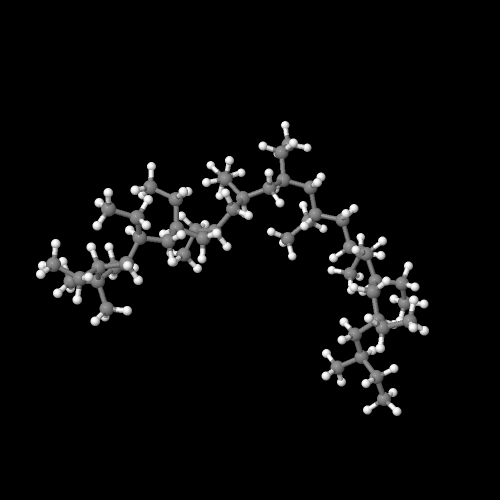
Linear polyethylene is normally produced with molecular weights in the range of 200,000 to 500,000, but it can be made even higher. Polyethylene with molecular weights of three to six million is referred to as ultra-high molecular weight polyethylene, or UHMWPE. UHMWPE can be used to make fibers which are so strong they replaced Kevlar for use in bullet proof vests. Large sheets of it can be used instead of ice for skating rinks.
Polyethylene is vinyl polymer, made from the monomer ethylene. Here's a model of the ethylene monomer. It looks like some sort of art nouveau teddy bear if you ask me.
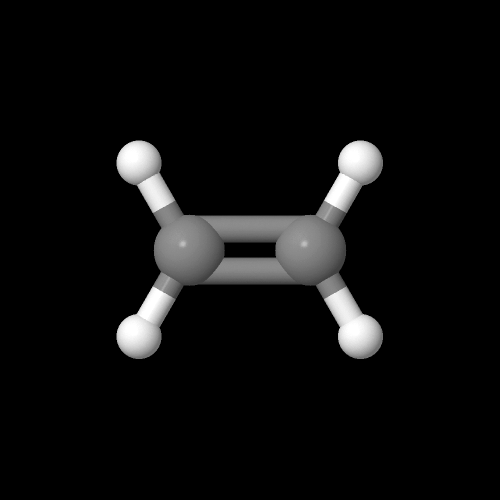
The model above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
Branched polyethylene is often made by free radical vinyl polymerization. Linear polyethylene is made by a more complicated procedure called Ziegler-Natta polymerization. UHMWPE is made using metallocene catalysis polymerization.
But Ziegler-Natta polymerization can be used to make LDPE, too. By copolymerizing ethylene monomer with a alkyl-branched comonomer such as one gets a copolymer which has short hydrocarbon branches. Copolymers like this are called linear low-density polyethylene, or LLDPE. BP produces LLDPE using a comonomer with the catchy name 4-methyl-1-pentene, and sells it under the trade name Innovex¨. LLDPE is often used to make things like plastic films.

| Other polymers used as plastics include: | Other polymers used as fibers include: |
| Polypropylene | Polypropylene |
| Polyesters | Polyesters |
| Polystyrene | Nylon |
| Polycarbonate | Kevlar and Nomex |
| PVC | Polyacrylonitrile |
| Nylon | Cellulose |
| Poly(methyl methacrylate) | Polyurethanes |

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |